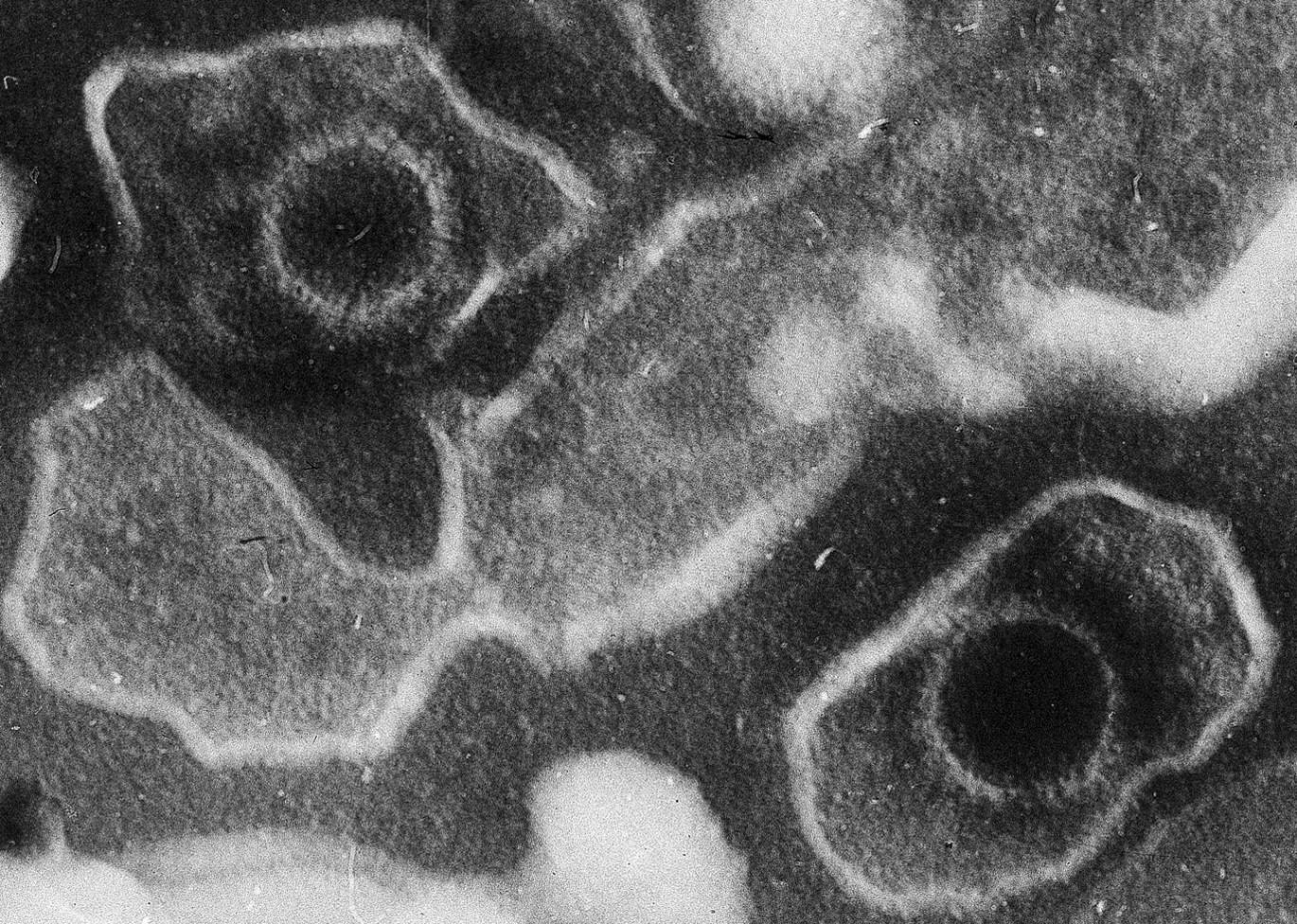
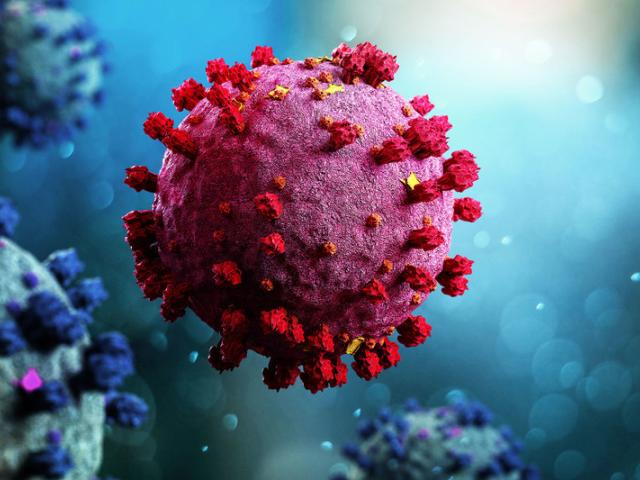
It hides in nearly everyone you know—inside your family, your friends, even yourself. The Epstein-Barr virus, or EBV, quietly lives within the cells of about 95% of adults worldwide. For most, it’s harmless, lying dormant after a fleeting illness. But for some, this seemingly silent passenger can awaken a devastating storm: lupus, one of the most mysterious and debilitating autoimmune diseases known to medicine.
Now, scientists at Stanford Medicine believe they’ve found the missing link connecting EBV to lupus. Their groundbreaking study, published in Science Translational Medicine, suggests that this common virus is not just associated with lupus—it may be the direct trigger that causes it. The discovery could transform how doctors understand, diagnose, and eventually prevent autoimmune diseases.
“This is the single most impactful finding to emerge from my lab in my entire career,” said Dr. William Robinson, professor of immunology and rheumatology at Stanford and the study’s senior author. “We think it applies to 100% of lupus cases.”
A Disease of the Body Turning Against Itself
Lupus—known formally as systemic lupus erythematosus—is a disease where the immune system, designed to protect us, turns traitor. Instead of attacking invading pathogens, it targets the body’s own tissues: skin, joints, kidneys, nerves, even the heart. The symptoms can vary widely, from fatigue and joint pain to life-threatening organ damage.
Around 5 million people globally live with lupus, and in the United States alone, several hundred thousand are affected. Strikingly, nine out of ten lupus patients are women—a disparity that continues to baffle scientists. Though modern treatments can help control the disease, there is no cure.
For decades, researchers suspected that lupus might begin with an infection that confuses the immune system. Among the candidates, Epstein-Barr virus has always stood out. But until now, no one could prove how this virus might ignite such chaos.
Meeting the Epstein-Barr Virus
Most of us encounter EBV in childhood or adolescence, often without realizing it. It spreads through saliva—shared utensils, a casual sip from someone’s drink, or a kiss. Sometimes it causes infectious mononucleosis, better known as “the kissing disease,” marked by fever and fatigue that can linger for months.
But the virus never truly leaves. Like a stowaway, EBV slips into the nuclei of our cells, embedding its genetic material and falling into a dormant state that can last a lifetime. The virus particularly favors B cells, a type of immune cell that produces antibodies to fight infection.
Normally, B cells help defend the body by recognizing foreign invaders. But they also have a darker side: around 20% of them are “autoreactive,” meaning they accidentally recognize and can attack the body’s own tissues. Thankfully, these self-targeting cells usually remain quiet—until something, or someone, wakes them up.
When the Virus Turns the Immune System Rogue
What the Stanford team discovered is that EBV can hijack the immune system’s control switch. When the virus infects a B cell, it sometimes produces a viral protein called EBNA2. This protein acts like a rogue engineer, flipping on genes that should have remained silent.
Two of these genes make powerful human proteins that, in turn, activate even more inflammation-related genes. The infected B cell becomes a kind of command center—an overzealous messenger broadcasting danger signals.
Those signals don’t just awaken nearby immune cells; they summon reinforcements. Other B cells, including the normally dormant autoreactive ones, suddenly activate and start producing antibodies that target the body’s own DNA and proteins inside cell nuclei. These “antinuclear antibodies” are the very markers doctors use to diagnose lupus.
In effect, the virus tricks the immune system into waging war against itself.
From One Cell to an Immune Army
The team used a revolutionary sequencing technology to find and analyze these infected B cells. In healthy people, only about one in every 10,000 B cells carries dormant EBV. But in people with lupus, that number skyrockets to one in 400.
That may sound small, but in the vast world of immune cells—hundreds of billions of them inside every person—this difference is enormous. Those few infected cells can ignite a chain reaction that mobilizes countless others. Once the self-directed immune attack begins, it can spread even to uninfected cells, perpetuating the cycle of autoimmunity.
When the army of autoreactive cells grows large enough, lupus symptoms begin to appear: rashes, fevers, fatigue, pain, and inflammation throughout the body.
Why Most People Don’t Get Lupus
If almost everyone carries EBV, why doesn’t everyone develop lupus? That’s the puzzle researchers are now working to solve.
Dr. Robinson suspects the answer lies in the virus’s genetic diversity. Not all strains of EBV are alike—some may be more likely to “flip the switch” that turns B cells into hyperactive, self-attacking machines. Genetics, environment, and even hormonal factors might also play roles in determining who is vulnerable.
It may also depend on timing. Early infection in childhood might train the immune system to tolerate EBV, while infection later in life—when the immune system is more reactive—could increase risk. For now, these ideas remain theories waiting for further research.
Beyond Lupus: A New Frontier in Autoimmune Research
The implications of this discovery stretch far beyond lupus. There’s growing evidence that Epstein-Barr virus could be involved in other autoimmune disorders such as multiple sclerosis, rheumatoid arthritis, and Crohn’s disease. In each, scientists have found traces of EBV’s genetic fingerprints in overactive immune cells.
If EBV truly acts as a spark for multiple autoimmune diseases, targeting it could change the future of treatment.
The Promise of an EBV Vaccine
An effective vaccine against EBV could prevent millions of future cases of lupus and other autoimmune conditions. Several pharmaceutical companies are now racing to develop one, with some vaccines already in clinical trials.
However, there’s a catch: vaccines can only prevent infection, not eliminate the virus once it’s established in the body. To protect people, an EBV vaccine would have to be given very early in life—possibly in infancy—before natural exposure occurs.
Still, the promise is enormous. If scientists can stop the virus before it hides within our immune cells, they might also stop the cascade of autoimmune diseases it can set in motion.
A Turning Point for Autoimmunity
For the millions who live with lupus, this discovery offers something rare: hope rooted in science. It transforms the way we think about autoimmunity—not as a random malfunction, but as a consequence of an ancient viral partnership gone wrong.
It also underscores a profound truth about the human body: that our greatest vulnerabilities often lie in the same systems that keep us alive. The immune system is a marvel of biological engineering, capable of learning, remembering, and defending. But when misled—by a virus, a mutation, or even stress—it can become its own worst enemy.
The Virus We All Carry
In a sense, the Epstein-Barr virus is a mirror of humanity itself—ancient, adaptable, persistent. It lives quietly within us, usually benign, sometimes dangerous. Most people will never feel its effects beyond a mild infection. But for a few, it rewrites the rules of the immune system entirely.
The discovery from Stanford doesn’t just illuminate a single disease—it reveals how deeply entwined our biology is with the invisible world of viruses. It reminds us that within our cells lies a history millions of years old, and that the line between friend and foe can be as thin as a single molecule of DNA.
More information: Shady Younis et al, Epstein-Barr virus reprograms autoreactive B cells as antigen presenting cells in systemic lupus erythematosus, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.ady0210