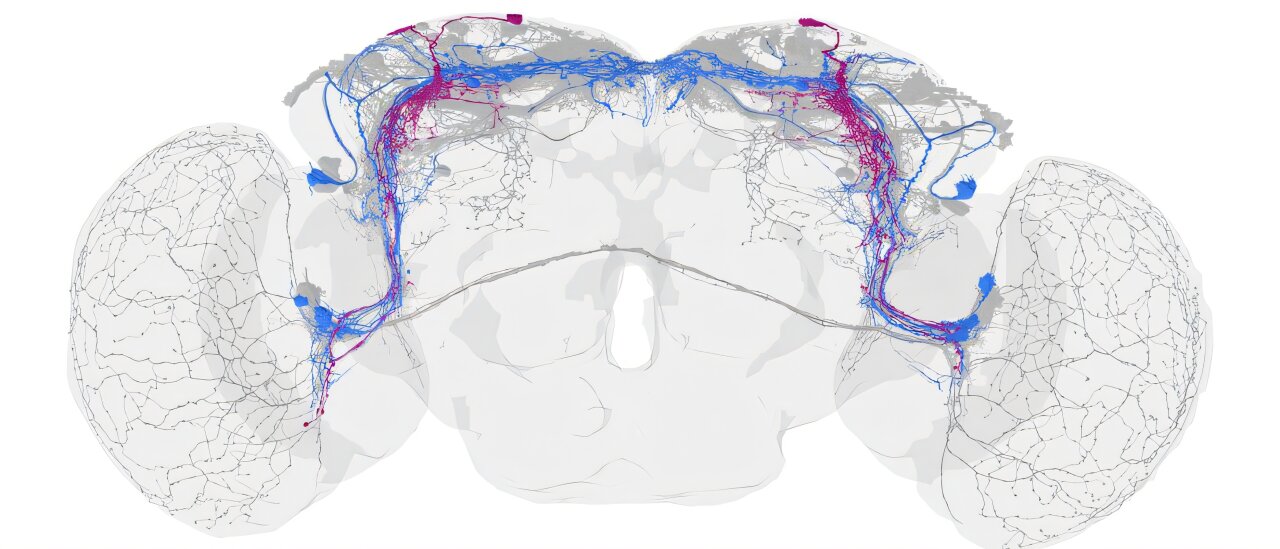
Inside the tiny brain of a fruit fly, time itself ticks to an astonishingly simple rhythm. For years, scientists believed that hundreds of neurons were needed to keep track of day and night in these small creatures. But new research from the University of Würzburg has revealed something extraordinary: just four specialized nerve cells—out of about 240 in the fly’s clock network—are enough to sustain its entire daily rhythm.
This discovery, published in the Proceedings of the National Academy of Sciences by Professor Charlotte Förster’s team, fundamentally reshapes our understanding of biological clocks. It shows that even the most complex systems in nature may arise from surprisingly minimal foundations.
The Universal Pulse of Life
Almost every living organism on Earth—from single-celled algae to humans—follows an internal rhythm that aligns with the 24-hour cycle of day and night. This is known as the circadian rhythm, a roughly daily pattern that governs when we sleep, eat, wake, and function.
In humans, it regulates body temperature, hormone levels, and metabolism. In animals, it determines when they forage or rest. Even plants open and close their leaves in response to it. These rhythms are not driven by sunlight alone—they are generated internally, through complex biological mechanisms fine-tuned over millions of years of evolution.
The fruit fly (Drosophila melanogaster) has long been one of the most important models for studying these rhythms. Its small brain contains around 240 so-called clock neurons, each housing molecular machinery that keeps time. Compared to humans, who have tens of thousands of such neurons, this network might seem simple. But until now, even the fly’s clock appeared too complex for researchers to determine which neurons truly drive the system.
The Mystery of the Master Clock
For decades, scientists debated which neurons in the fruit fly brain serve as the “master pacemaker”—the central conductor that synchronizes all others. Earlier studies pointed to certain neurons on the sides of the brain, believed to coordinate the timing signals for the rest. But these conclusions rested on indirect evidence. The internal dialogue between neurons in the clock network remained largely a mystery.
Dr. Nils Reinhard and his colleagues at the University of Würzburg decided to test the system in a radically simplified way. If they could silence most of the clock neurons, they reasoned, they might see whether a smaller group could still keep time. What they discovered went far beyond anyone’s expectations.
When Four Neurons Keep Time
The researchers genetically silenced 236 of the fruit fly’s 240 clock neurons, effectively switching off their internal clocks. Only four specific neurons, two in each hemisphere of the brain, were left functioning.
What happened next astonished the scientists. Despite losing nearly its entire network, the fruit fly continued to follow a normal daily rhythm. It remained active during the morning and evening—just as healthy flies do—and this rhythmic behavior persisted even when the environment provided no external light cues.
Dr. Reinhard summarized the result succinctly: “These four neurons alone were sufficient to maintain the fly’s basal activity rhythm.”
In other words, these few neurons formed a core circuit—a miniature pacemaker that could sustain the fundamental rhythm of life on its own.
Two Messengers, Two Moments
The Würzburg team went even further. They discovered how these four neurons manage to control the timing of activity throughout the day. Each neuron communicates using chemical messengers—neurotransmitters—that send signals to other parts of the brain.
For the morning activity, the neurons use a molecule called CCHamide-1 (CCHa1). For the evening phase, they rely on the well-known neurotransmitter glutamate. This dual signaling system allows the tiny network to distinguish between different times of day, acting like a miniature chemical orchestra that plays two separate melodies—one for dawn, another for dusk.
The discovery of these two messenger systems provides a detailed blueprint of how biological clocks can be built from the simplest components. Even with just four neurons, the system maintains both flexibility and precision—two essential qualities for any organism trying to survive in a world of light and darkness.
A Glimpse Into Evolution’s Blueprint
One of the most intriguing aspects of this study comes from developmental biology. The team discovered that these four neurons belong to a set of 18 neurons that already exist in the larval stage of the fruit fly. As the fly matures, these same neurons persist into adulthood, forming the backbone of the adult circadian system.
This means that the essential machinery of timekeeping is established very early in life, long before the fly develops its full neural complexity. As the animal grows, more neurons are added—not to replace the original core, but to refine and expand it.
Dr. Reinhard described this as a “core clock” system: a primitive but complete network that lays the foundation for all later circadian behavior. The additional neurons that appear during development don’t create the rhythm; instead, they fine-tune it, allowing the fly to respond more sensitively to environmental changes such as light, temperature, and seasonal shifts.
This idea paints a picture of evolution as an architect that starts with simplicity and builds complexity on top of it—a pattern seen throughout biology.
Connecting Competing Theories
For years, chronobiologists have debated between two models of how biological clocks are organized. One model proposed a hierarchical system, where a dominant pacemaker dictates the timing for all others. The other suggested a distributed network, where timing arises from cooperation among many neurons without a clear leader.
The Würzburg study elegantly bridges these two ideas. It shows that both models are correct—but at different levels. At its heart, the clock has a hierarchical core of key neurons that generate the fundamental rhythm. Around this core, a flexible network of additional neurons modulates and synchronizes timing with the external world.
This dual structure—simple at its center, complex at its edges—may not only apply to fruit flies but could represent a universal design principle for biological timekeeping across species.
From Flies to Humans: The Broader Implications
While the study focuses on fruit flies, its implications extend much further. Biological clocks in humans operate on the same basic molecular principles. In mammals, the suprachiasmatic nucleus (SCN)—a small cluster of about 20,000 neurons in the brain—acts as our central pacemaker.
Just as in flies, new neurons appear in the mammalian clock during development. Interestingly, in humans, the clock’s structure also changes during puberty, when the timing of sleep and activity shifts naturally. These parallels suggest that the concept of a core clock that expands over time could be a common theme across evolution.
Understanding this minimal timekeeping network could one day help scientists uncover why circadian rhythms sometimes go awry in humans, leading to sleep disorders, depression, metabolic problems, and even cancer. It may also inspire new ways to design therapies that restore or regulate the body’s natural rhythms.
The Power of Simplicity in Complexity
There is something deeply poetic in the idea that such a fundamental function as the perception of time can arise from just four neurons. In a world filled with complexity—where countless cells and molecules interact in bewildering patterns—this discovery offers a humbling reminder of nature’s efficiency.
Biological systems often achieve sophistication not by adding endless layers of parts, but by arranging a few components in elegant ways. The fruit fly’s internal clock is a perfect example of this principle: a tiny circuit that keeps perfect time in an ever-changing world.
More information: Nils Reinhard et al, A functional clock in only two dorsal clock neurons is sufficient to restore the basal circadian activity pattern of Drosophila melanogaster, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2506164122