At first glance, prolinol looks like a modest character in the vast world of chemistry. It is small, flexible, and familiar to chemists who use it as a catalyst and as a building block in chemical synthesis. Yet when researchers began watching what happens as water molecules gather around it, prolinol revealed a surprisingly dramatic side. With the arrival of just a handful of water molecules, its entire preferred shape shifted, as if the molecule were quietly rewritten by hydration itself.
This discovery, reported in the Journal of the American Chemical Society, did not come from studying oceans of liquid water or crowded biological environments. Instead, it came from zooming in to an almost intimate scale, where one, two, or three water molecules are enough to tip the balance. The result is a vivid reminder that in chemistry, even the smallest interactions can have outsized consequences.
The Mirror-Image Puzzle at the Heart of Chemistry
To understand why this matters, it helps to step into the world of physical chemistry, where scientists use the tools of physics to explain how matter behaves at the molecular level. One long-standing question in this field is how molecules change their structure as they interact with their surroundings, especially during chemical reactions.
This question becomes particularly intriguing when chiral molecules enter the picture. Chiral molecules are like left and right hands. They are made of the same parts, yet they cannot be placed on top of their mirror image in a way that makes them match. This subtle asymmetry has enormous consequences, influencing how molecules recognize each other, how they react, and how they function in chemical and biological systems.
Water, ever-present and often taken for granted, plays a central role here. Understanding how chiral molecules interact with water is crucial, because water is the stage on which much of chemistry and biology unfolds. The mystery is not whether water matters, but how, exactly, it exerts its influence at the smallest scales.
Bringing Prolinol Under the Microscope
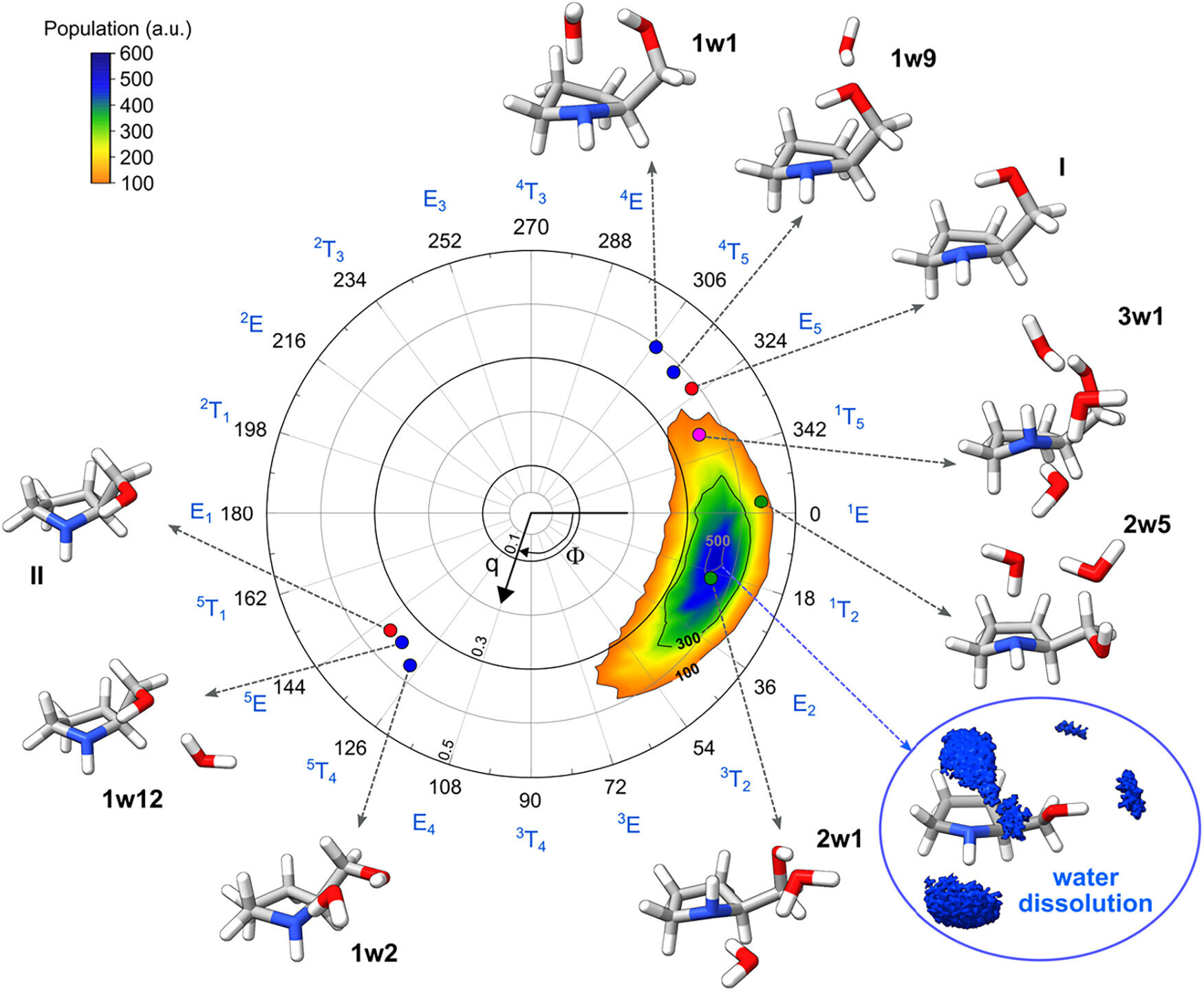
This is where the Spectroscopy Group of the University of the Basque Country (EHU) and the Biofisika Institute (CSIC/EHU) stepped in. Led by Emilio J. Cocinero, the team set out to observe prolinol as it interacted with water in the most controlled way imaginable. Instead of immersing it in bulk liquid, they added water molecules one by one, carefully studying the resulting structures.
Prolinol was an ideal choice for this kind of investigation. It is not only widely used in asymmetric synthesis, but it also carries two functional groups, an alcohol and an amine, both of which interact strongly with water. These groups create a delicate internal balance within the molecule, held together by internal bonds that determine its preferred shape.
What makes prolinol especially revealing is its flexibility. Its structure can bend and twist, making it sensitive to outside influences. This means that when water arrives, its effects do not stay hidden. They show up clearly, reshaping the molecule in ways that can be directly observed.
Watching Water Take Control, One Molecule at a Time
As the researchers began adding water molecules to prolinol, something striking happened. With each step of hydration, the molecule responded, adjusting its conformation. When just one water molecule attached itself, subtle changes appeared. With two, the balance shifted further. By the time three water molecules were involved, the preferred structure of prolinol had changed completely.
This was not water acting as a passive background. It was water taking an active role, competing with the molecule’s internal bonds and, in some cases, overpowering them. Structures that would normally be considered unfavorable suddenly became preferred, all because of a few carefully placed water molecules.
Cocinero described this effect with striking clarity. Water, he explained, acts as a true conformational switch. By forming interactions with the alcohol and amine groups, water can force prolinol into shapes it would not adopt on its own. The molecule’s identity remains the same, but its behavior and potential reactivity are transformed.
Connecting Three Worlds That Rarely Meet
One of the most powerful aspects of this study lies in how it bridges scales that are usually examined separately. Researchers often study molecules in complete isolation, far removed from any environment. Others focus on molecules fully dissolved in solution, surrounded by countless solvent molecules. The space in between, where only a few solvent molecules are present, is harder to explore and often overlooked.
This work connects all three. It links the isolated molecule, the microsolvation stage involving just a few water molecules, and the behavior observed in solution. According to Cocinero, this connection is essential. By understanding how hydration begins, scientists can better understand what eventually happens in the complex environments of real-world chemistry.
To achieve this, the team used an experimental approach that combined ultra-high-resolution rotational spectroscopy with theoretical calculations, as well as studies in solution using NMR and simulations. This combination allowed them to see, in extraordinary detail, how prolinol responds as water molecules gather around it and how those early changes echo in solution.
When Less Water Tells You More
One of the most surprising messages from the study is how little water it takes to learn something profound. It might seem intuitive that the behavior of molecules in water can only be understood by studying them in fully hydrated environments. Yet this research suggests the opposite can sometimes be true.
By closely examining what just two or three water molecules do, the researchers gained insights into how prolinol behaves in solution. These few molecules were enough to reveal the key interactions that drive structural changes. They acted like messengers, carrying the essential information about how water reshapes the molecule’s internal landscape.
This idea challenges assumptions about scale. It suggests that for many chemical and biological systems, the earliest moments of hydration may already contain the answers scientists are looking for. Understanding these moments can provide clarity that might otherwise be lost in the complexity of bulk solution.
Why This Quiet Molecular Drama Matters
At the end of this story, the importance of the findings comes into focus. The conformation of a molecule determines how it recognizes others, how it reacts, and how it functions in its environment. When water can completely change that conformation with just a few molecules, the implications ripple outward.
For chemistry, this deepens our understanding of how catalysts like prolinol behave in realistic conditions. For physical chemistry, it reinforces the idea that water is not merely a solvent, but an active participant that shapes molecular identity. For anyone interested in the foundations of chemical and biological processes, it offers a powerful lesson: sometimes, the most meaningful transformations happen quietly, one molecule at a time.
By revealing water as an active force and showing how microscale interactions connect to behavior in solution, this research opens a clearer window into the molecular world. It reminds us that even the smallest encounters can redefine structure, function, and possibility, and that by paying attention to these subtle shifts, we can better understand the chemistry that underlies the world around us.
Study Details
Donatella Loru et al, Stepwise Hydration Reveals Conformational Switching in Chiral Prolinol, Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.5c13582