Beneath the eternal snow of the Tibetan mountains, deep within Finland’s frozen groundwater, and on the windswept glaciers of Greenland, life clings to survival in some of the coldest corners of Earth. To most, these landscapes are the realm of stillness, silence, and icy beauty. But to one scientist—Kirill Kovalev—they are a hidden galaxy of possibility.
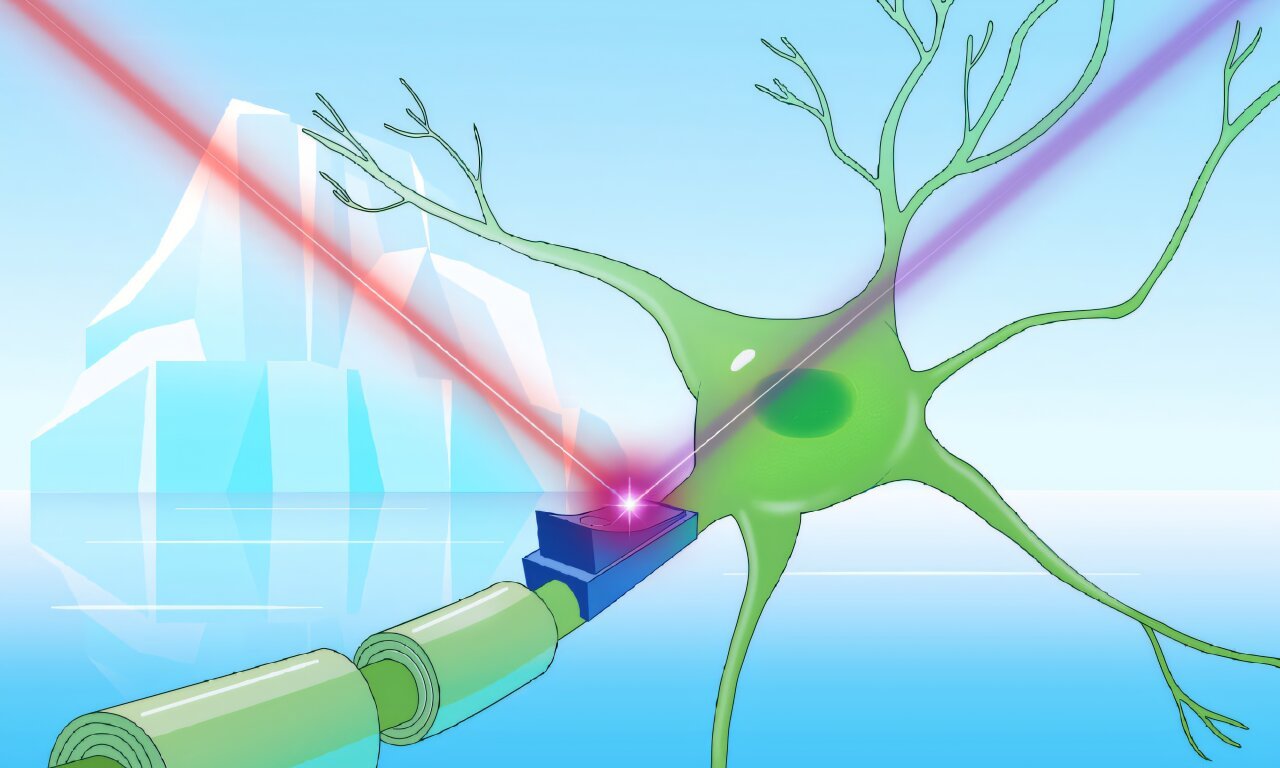
Kovalev, a physicist-turned-structural-biologist and EIPOD Postdoctoral Fellow at EMBL Hamburg and EMBL-EBI, sees these harsh habitats not as desolate but as vibrant frontiers of molecular innovation. And it’s here, in the biological remnants of ice-bound microbes, that he’s discovered something remarkable: a family of mysterious proteins that could one day help scientists switch brain cells on and off—with beams of light.
The molecules are called cryorhodopsins—rare and resilient proteins from cold-loving microbes. But these are no ordinary proteins. Some of them shimmer blue, and their light-sensitive properties may one day revolutionize neuroscience, medicine, and biotechnology.
Welcome to the story of how frozen microbes may hold the key to turning light into thought.
A Serendipitous Discovery in a Cold World
Like many scientific revelations, Kovalev’s journey began with curiosity and chance. One day, while exploring a database of microbial proteins, something peculiar caught his attention. A handful of rhodopsins—proteins normally found in aquatic environments like lakes and oceans—were showing up in the genomes of microbes from cold places: glaciers, alpine peaks, and frozen groundwater.
“That’s weird,” he thought.
But the strangeness didn’t stop there. These proteins shared a striking similarity across microbes separated by thousands of kilometers. Such molecular conservation usually signals one thing: survival. Whatever these proteins were doing, they were doing something essential in the frigid extremes of Earth.
Driven by instinct and wonder, Kovalev gave them a name that acknowledged their frosty habitat: cryorhodopsins—from the Greek “kryos” for cold.
Color as a Clue to Power
To understand cryorhodopsins, you first need to understand rhodopsins in general. These colorful proteins are nature’s solar panels. Found in microorganisms and even human eyes, they absorb specific wavelengths of light to power vital biological processes.
Most rhodopsins wear a pink-orange hue, reflecting those warm colors and absorbing light from the green and blue spectrum. But scientists have long searched for rhodopsins of other colors—especially blue ones—because they respond to longer-wavelength red light, which penetrates living tissue more deeply and non-invasively. Blue rhodopsins could become powerful tools in optogenetics—a technique that uses light to activate or deactivate brain cells in real time.
So when Kovalev shined light on cryorhodopsins in his lab, what he saw amazed him: a rainbow of responses. Some cryorhodopsins turned out to be blue.
The colors weren’t just aesthetic. They told a story about the molecular structure of each protein—how it bends light, how it behaves, and, most crucially, how it might be engineered for science.
“I can actually tell what’s going on with a cryorhodopsin simply by looking at its color,” Kovalev said.
Using advanced techniques such as X-ray crystallography and cryo-electron microscopy, he found that a rare structural twist was responsible for the blue color. This twist, spotted originally in the digital data, was now staring at him in high-resolution structural detail. For a physicist chasing biological mysteries, it was a eureka moment.
And it meant something profound: Now that scientists know what makes rhodopsins blue, they can design them.
From Ice to Brain: A New Tool for Optogenetics
Armed with this insight, Kovalev and his collaborators took their work a step further. They tested how cryorhodopsins behave in real, living cells—specifically, cultured brain cells. What happened next was nothing short of astonishing.
When the cells expressing cryorhodopsins were exposed to ultraviolet (UV) light, they lit up—electrical currents surged through them. But when the cells were immediately exposed to green light, their excitability increased. If the team used red or UV light instead, the excitability dropped.
This dual-mode response—being able to turn a neuron’s activity both “on” and “off” with different colors of light—is the holy grail of optogenetics.
“New optogenetic tools to efficiently switch the cell’s electric activity both ‘on’ and ‘off’ would be incredibly useful in research, biotechnology, and medicine,” said Tobias Moser, a neuroscientist at the University Medical Center Göttingen and co-author on the study.
Moser’s lab is working on futuristic cochlear implants that use light, rather than electricity, to restore hearing. Imagine being able to stimulate precise sound frequencies in deaf patients by shining tailored wavelengths of light inside the ear. Tools like cryorhodopsins could make this possible.
Kovalev, however, is careful not to overstate the readiness of cryorhodopsins as clinical tools.
“They’re not ready to be used yet,” he said. “But they’re an excellent prototype.”
A Microbial UV Alarm System?
As they delved deeper, Kovalev’s team began to suspect that cryorhodopsins weren’t just molecular light switches—they might also act as UV sensors, helping cold-dwelling microbes detect and avoid dangerous radiation.
In collaboration with Josef Wachtveitl’s group at Goethe University Frankfurt, the researchers discovered that cryorhodopsins are unusually slow to respond to light. This sluggishness isn’t a flaw—it might be a feature. In nature, it could allow microbes to track sustained exposure to harmful UV rays.
But sensing light is only half the story. To act on the signal, a microbe needs a messenger—a molecule that can relay the information deeper into the cell.
Kovalev noticed that wherever the gene for cryorhodopsin appeared, there was always another gene tagging along—one encoding a tiny, mysterious protein of unknown function. Could this be the missing link?
Using AlphaFold, an AI tool that predicts protein structure, Kovalev’s team revealed that five copies of this small protein likely form a ring-shaped complex that nestles next to the cryorhodopsin inside the cell. They believe that when UV light is detected, this ringed protein might disengage, carrying the signal onward.
“It was fascinating to uncover a new mechanism via which the light-sensitive signal from cryorhodopsins could be passed on,” said co-supervisor Alex Bateman from EMBL-EBI.
And intriguingly, this mysterious protein isn’t limited to microbes with cryorhodopsins. It shows up elsewhere in nature, hinting at a broader biological role yet to be uncovered.
Evolution’s Arctic Gift to Science
Why did cryorhodopsins evolve only in cold places? And why are they so uniquely structured?
The current theory is that UV radiation, rather than cold itself, was the evolutionary driver. High-altitude and polar regions are notorious for intense UV exposure. For microbes living there, survival may have depended on evolving a UV alert system—and cryorhodopsins may be exactly that.
It’s another example of how nature, when pushed to the edge, invents extraordinary solutions. And if scientists are paying attention, they can learn from it—not just about biology, but about potential breakthroughs in human health and technology.
“Discovering extraordinary molecules like these wouldn’t be possible without scientific expeditions to often remote locations, to study the adaptations of the organisms living there,” Kovalev said. “We can learn so much from that.”
A Symphony of Light, Ice, and Insight
To decode the secrets of cryorhodopsins, Kovalev and his team had to bring together a rare blend of techniques: crystallography, cryo-EM, artificial intelligence, neuroscience, and a bit of old-fashioned patience.
The EMBL Hamburg beamline P14, uniquely suited for Kovalev’s project, allowed the team to analyze proteins activated by light with atomic precision. In Groningen, Netherlands, collaborators helped refine cryo-EM studies. Across Europe, scientists worked with samples in near-total darkness to prevent accidental light activation.
“This project was only possible because of the incredible support and collaboration across institutes,” said Kovalev. “The whole P14 beamline team worked together to tailor the setup to my experiments. I’m very grateful.”
What began with a digital hunch in a protein database has grown into a continental scientific endeavor—and a thrilling glimpse into how ice-age proteins could become tomorrow’s neural tools.
From Frozen Microbes to the Future of Medicine
Kovalev’s discovery is a reminder of science’s most enduring truth: the world still holds secrets in its most remote corners.
Cryorhodopsins are more than a novel protein family—they are a bridge between nature’s ingenuity and human invention. One day, they might be used to repair sight, restore hearing, regulate pain, or treat neurological disorders.
They began in ice, but they may end in saving minds.
As Kovalev put it: “Every time we learn how life survives in extreme conditions, we gain not just knowledge—but new tools, new questions, and sometimes, a whole new way to see the world.”
In a silent glacier, a blue flash flickers. And in that cold light, a future begins.
Reference: Gerrit Lamm et al, CryoRhodopsins: a comprehensive characterization of a group of microbial rhodopsins from cold environments, Science Advances (2025). DOI: 10.1126/sciadv.adv1015. www.science.org/doi/10.1126/sciadv.adv1015