In the quiet chambers of a Texas lab, a daring experiment unfolded. Scientists at UT Southwestern Medical Center asked a question so bold it verged on biological heresy: What happens if we take away a cell’s mitochondria—its powerhouses, its dynamos of energy, its evolutionary time machines?
The answer, it turns out, is not death. At least, not right away. Some cells adapt. Some survive. And in their survival, they whisper secrets about our evolution, our brains, our aging—and even the possibilities for healing some of the most devastating genetic diseases known to medicine.
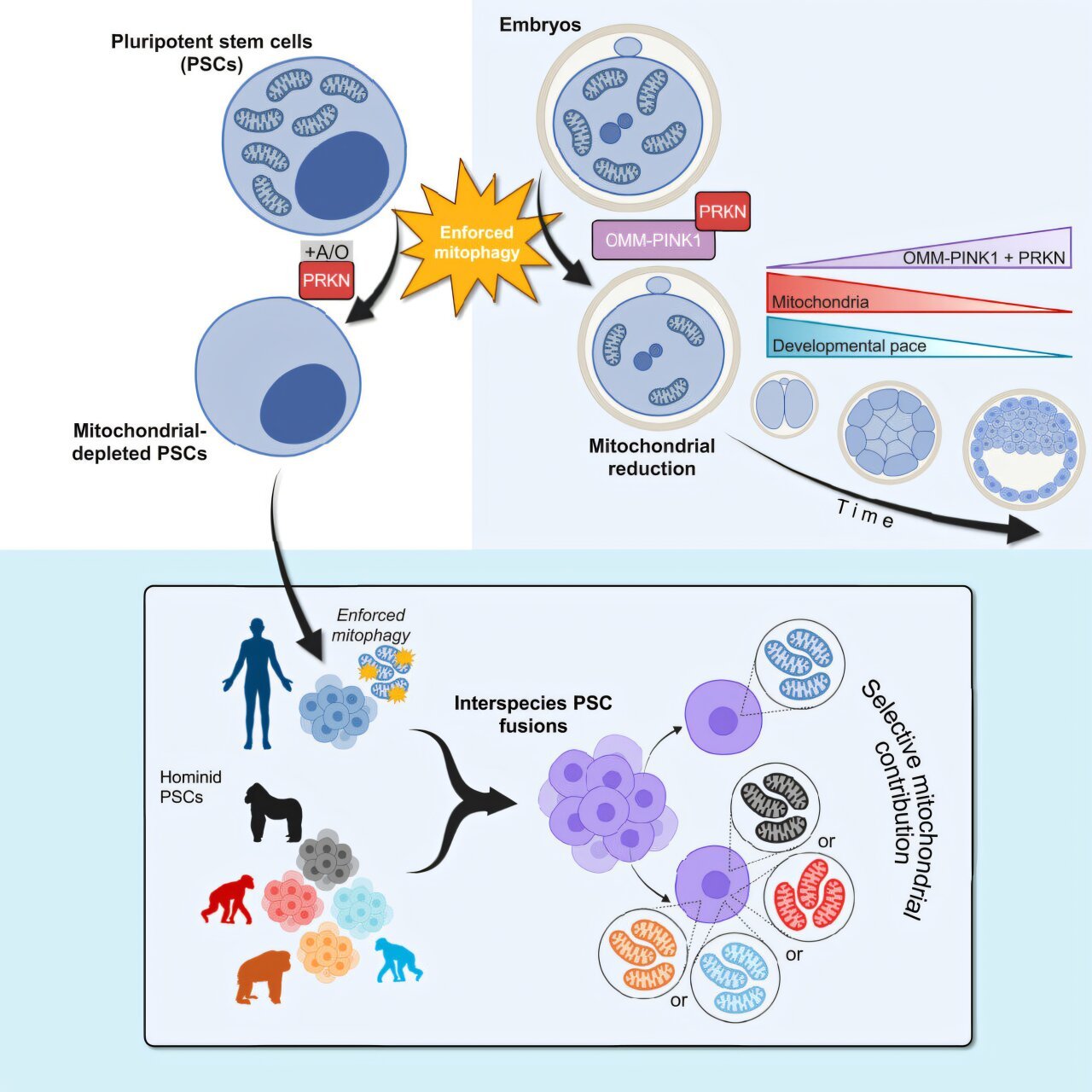
Now published in the prestigious journal Cell, this groundbreaking research—led by Dr. Jun Wu and Dr. Daniel Schmitz—offers a revolutionary new tool: enforced mitophagy, a genetic technique that prompts cells to strip away every last mitochondrion they possess. And by forcing cells to operate without these iconic organelles, the scientists uncovered how mitochondria communicate with the cell nucleus, how they influence gene expression, and how they might shape the very essence of what makes us human.
The Silent Conversation Between Past and Present
Mitochondria are more than just biological batteries. They’re relics of an ancient alliance forged more than a billion years ago when a single-celled ancestor of all modern eukaryotes absorbed a free-living bacterium. Rather than digest it, the cell let it stay. That bacterium evolved into the mitochondrion, and the two entities became one—forever linked in a biological pact.
But mitochondria didn’t give up all their independence. They retained their own DNA, passed exclusively through mothers, and established a mysterious dialogue with the nucleus—an intricate crosstalk that scientists have long struggled to decode.
“What happens when that conversation stops?” Dr. Wu asked.
Until now, there was no way to find out. But enforced mitophagy has changed that. By hijacking the cell’s natural process for disposing of damaged mitochondria, the researchers created a method for emptying cells of mitochondria altogether. Then they listened—to the chaos, to the adaptation, to the silence left behind.
Mitochondria Gone, Cells Survive
To begin, the team tested enforced mitophagy on human pluripotent stem cells (hPSCs), which can develop into nearly any cell type in the body. When stripped of their mitochondria, these early-life cells stopped dividing—but astonishingly, they didn’t die. For up to five days, they held on, floating in petri dishes as if waiting for a new set of instructions.
Similar resilience was observed in mouse stem cells and even in hPSCs with known mitochondrial mutations. In other words, this was no fluke. Cells across species—and even those with diseased mitochondria—could survive temporary mitochondrial exile.
And that raised even bigger questions: How? What kept them alive? And what were they doing instead?
Genes Begin to Whisper
Using advanced techniques to measure gene expression, the researchers dove into the nuclear responses of these mitochondria-depleted cells. Out of thousands of genes, 788 dialed down their activity. But 1,696 became more active—as though the cell, deprived of its engines, was rewiring its circuits to compensate.
Some of the activated genes encoded proteins that took over energy production and regulatory roles usually played by mitochondria. It was a biological improvisation. A backup plan. A testament to the cell’s incredible plasticity.
More profoundly, the cells seemed to retain their identity. They could still, at least on paper, become other types of cells. Life, it appeared, could still find a way.
Human Versus Primate: The Mitochondrial Mirror
Then came one of the most imaginative steps of the study.
Dr. Wu’s team fused human stem cells with stem cells from our closest evolutionary cousins—chimpanzees, bonobos, gorillas, and orangutans. These hybrid “composite” cells contained two nuclear genomes and two sets of mitochondria: one human, one primate.
What happened next was astonishing. The cells systematically eliminated the non-human mitochondria. They chose human over chimpanzee. Human over bonobo. As though some internal compass favored one over the other.
But the researchers weren’t done.
In a follow-up experiment, they reversed the setup. This time, they stripped the human cells of mitochondria first, then fused them with non-human primate stem cells—creating cells that housed both nuclear genomes but only non-human mitochondria.
The results? Almost no major disruptions. Despite millions of years of evolutionary separation, human nuclei and primate mitochondria worked together just fine. Most gene expression stayed stable. Only a handful of differences emerged—and intriguingly, many of them involved genes linked to brain development and neurological disorders.
“It raises an extraordinary possibility,” said Dr. Wu. “That mitochondria might play some role in shaping the evolution of the human brain.”
When Cells Fail to Grow: The Embryo Test
But cells in petri dishes are only part of the story. Could this enforced mitophagy technique reveal how mitochondria shape development in a whole organism?
To find out, the team engineered mouse embryos with reduced mitochondrial numbers, then implanted them into surrogate mothers. The results were stark. Embryos missing more than 65% of their mitochondria failed to implant entirely. Those missing about one-third showed developmental delays—but caught up over time.
That means mitochondria are not just energy producers. They’re developmental gatekeepers. There’s a threshold of mitochondrial abundance required for life to begin its journey—and below that, the spark of development falters.
A New Frontier for Mitochondrial Disease and Evolutionary Biology
For patients with mitochondrial diseases—like Leigh syndrome or Kearns-Sayre syndrome, which can ravage organs from the brain to the heart—this research may offer new hope. If scientists can learn how cells compensate for mitochondrial loss, or how to eliminate diseased mitochondria selectively, they may unlock new treatment strategies that were once considered science fiction.
But beyond medicine, these findings reach into the philosophical.
Mitochondria may help define what it means to be human—not just in our biology, but in our brains, our thoughts, our capacity for memory, music, and madness. The subtle differences between our mitochondria and those of our primate cousins may be tiny molecular switches that shape cognition, aging, and our species’ unique trajectory.
The Tool That Lets Cells Whisper
Enforced mitophagy isn’t just a technique—it’s a key. A key to understanding how cells manage their power supply. A key to decoding how organelles and nuclei coordinate across evolutionary time. A key that might one day allow us to reprogram diseased cells, redesign developmental pathways, or even, perhaps, slow the clock of aging.
“This is just the beginning,” said Dr. Schmitz. “We now have a tool to ask questions we’ve never been able to ask before.”
In a world obsessed with power, mitochondria have always held a special place—tiny engines with an ancient soul. But now, thanks to this bold experiment, we know something even more profound: cells remember, adapt, and survive even when their powerhouses go dark.
And in that flicker of resilience, we see the future of biology—a field no longer bound by what is, but by what might be.
Reference: Daniel A. Schmitz et al, Unraveling mitochondrial influence on mammalian pluripotency via enforced mitophagy, Cell (2025). DOI: 10.1016/j.cell.2025.05.020