Pancreatic cancer is often called a silent killer. It grows quietly, spreads aggressively, and resists most treatments, making it one of the deadliest cancers in the world. Scientists have long struggled to understand why this particular cancer is so ruthless. Now, groundbreaking research from the Technical University of Munich (TUM) has uncovered a chilling clue: pancreatic tumors can hijack the body’s nervous system to fuel their own growth.
In a study published in Cancer Cell, researchers led by Professor Ekin Demir have discovered that pancreatic tumors form special nerve-like connections known as “pseudosynapses.” These structures allow cancer cells to communicate directly with nerve cells, essentially tricking the body’s own electrical network into supporting the tumor. It’s as if the cancer plugs itself into the body’s wiring and starts using it for power.
The Discovery of Pseudosynapses
For decades, scientists have known that the nervous system can influence cancer development. Tumors often invade nearby nerve fibers—a phenomenon known as “neural invasion”—which usually signals an especially poor prognosis. But the new study takes this relationship to a deeper, more intimate level.
Around six years ago, American researchers studying brain tumors made a surprising discovery: some brain cancers could form their own synapses, the specialized connections neurons use to communicate. These tumor synapses allowed cancer cells to “listen in” on the brain’s electrical activity and use it to grow faster.
Professor Demir and his team wondered whether this strange mechanism could also occur outside the brain—particularly in organs where nerves are already deeply involved in regulating function. The pancreas was an ideal candidate. It is richly connected to the nervous system and known for frequent neural invasion by tumors.
Seeing the Invisible: Searching for Tumor Synapses
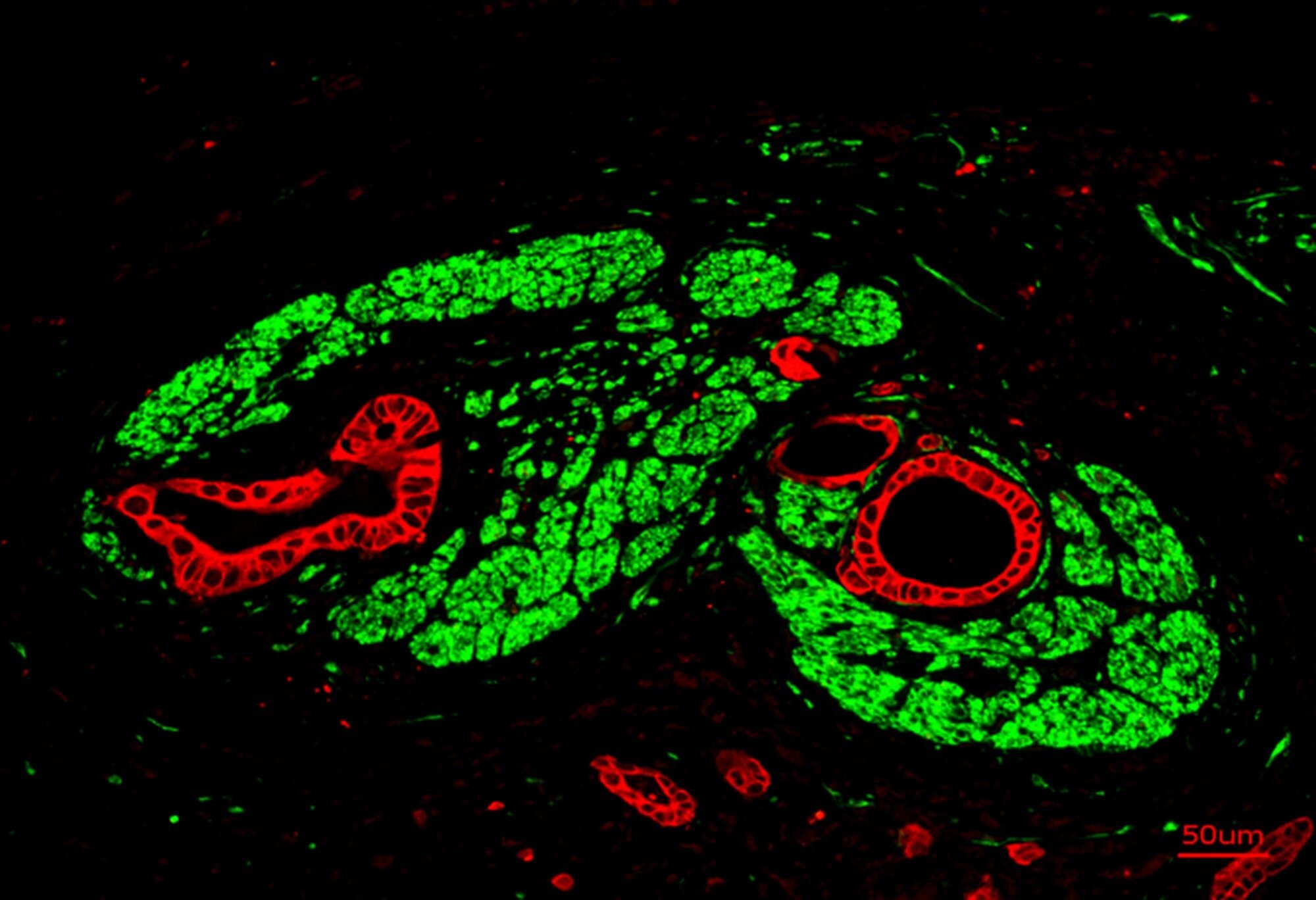
The TUM researchers began their search by studying pancreatic tumor tissue under high-resolution microscopes. They were looking for the molecular fingerprints of synapses—clusters of receptors that respond to neurotransmitters, the chemical messengers of the nervous system.
Their investigation paid off. In several samples, the scientists found unusually high concentrations of NMDA receptors—proteins that bind to glutamate, a neurotransmitter that plays a vital role in learning and memory in the brain. The presence of these receptors hinted that something extraordinary was happening.
When the team examined the tissue under an electron microscope, they saw tiny structures resembling synapses. These connections were not identical to true neuronal synapses but were close enough to perform a similar function. Because of their subtle structural differences, the researchers named them “pseudosynapses.”
These pseudosynapses, it turned out, were the cancer’s secret weapon.
How Cancer Exploits the Nervous System
The pancreas, like other glands, normally responds to signals from the nervous system to regulate its function. Under healthy conditions, glutamate molecules released by nerve cells bind to NMDA receptors on pancreatic cells, triggering a wave of calcium ions to enter the cell. This calcium flow sets off a chain reaction of signals that control normal cell activity.
Pancreatic tumors appear to have stolen this mechanism for their own advantage. When glutamate binds to NMDA receptors on cancer cells, it opens a channel that allows calcium to flood in. This influx sets off molecular signaling cascades that push the cancer cells to grow, divide, and spread.
Professor Demir describes it this way: “When glutamate binds to the cancer cells’ NMDA receptors, a channel opens and calcium flows into the cell. This influx triggers molecular signaling cascades that drive tumor growth and metastasis.”
The researchers noticed that these cancer-induced calcium waves were slow and long-lasting—very different from the quick bursts seen in normal nerve signaling. The sustained waves may help explain why pancreatic tumors are so persistent and aggressive.
Turning Discovery into Hope
While this discovery highlights how cunning cancer can be, it also points to a potential vulnerability. If tumors depend on these pseudosynapses and NMDA receptors for growth, then blocking this communication could slow or even stop the disease.
In experiments with mice, the TUM team tested drugs that inhibit NMDA receptors. The results were promising: the tumors grew more slowly, spread less often, and the animals lived longer.
This breakthrough opens the door to new therapies that target the interaction between nerves and cancer cells—a frontier that has barely been explored in oncology.
“We are currently using bioinformatic methods to identify approved drugs that, in addition to their primary effects, can also block these specific NMDA receptors in pancreatic cancer cells,” says Professor Demir. “Therapies targeting the interface between the nervous system and tumors could open up entirely new treatment options.”
Such drugs might not only treat pancreatic cancer but also other types of tumors that exploit the same nerve-based mechanisms.
A Broader Implication: When Cancer Talks to the Brain
The discovery that tumors can form pseudosynapses challenges how we think about cancer as a disease. Traditionally, we view tumors as isolated masses of rogue cells. But this research suggests that cancers may be integrated into the body’s own communication networks—using neurotransmitters, hijacking nerve signals, and reshaping normal physiology to favor their survival.
In other words, cancer may not only feed on nutrients and blood—it may also feed on information. By forming pseudosynapses, pancreatic tumors become active participants in the nervous system, blurring the line between body and disease.
This revelation could change how we approach treatment. Instead of targeting cancer cells alone, future therapies may focus on the “conversation” between the tumor and the body’s nerves—interrupting the chemical dialogue that fuels disease.
The Human Side of Discovery
Behind the technical language and microscopic details lies a story of human hope. Pancreatic cancer currently has one of the lowest survival rates of any cancer, partly because it is often detected too late and resists standard treatments like chemotherapy. For patients and their families, even small advances bring immense promise.
The discovery of pseudosynapses doesn’t just deepen our understanding—it opens a path forward. It suggests that hidden within the body’s own systems may lie keys to stopping the most untreatable cancers.
By understanding how tumors manipulate neural communication, scientists may one day silence the messages that drive them—and in doing so, give patients time, healing, and life.
The Next Chapter in Cancer Science
The TUM team’s findings mark a significant leap in the rapidly growing field of “cancer neuroscience,” which explores the connections between tumors and the nervous system. The idea that cancer cells might “talk” to neurons was once unthinkable, yet it is now reshaping scientific understanding across multiple cancers, from brain tumors to prostate and breast cancers.
The researchers believe that pseudosynapses could be more widespread than previously imagined. If other tumor types are found to use the same strategy, this mechanism could represent a universal vulnerability—one that scientists can exploit to design more precise, nerve-targeted cancer therapies.
As research continues, one thing becomes clear: the boundary between the nervous system and cancer is not as firm as once thought. Within that blurred line lies both danger and opportunity—the danger of cancer’s cunning adaptability, and the opportunity to outsmart it with science.
More information: Lei Ren et al, Sensory neurons drive pancreatic cancer progression through glutamatergic neuron-cancer pseudo-synapses, Cancer Cell (2025). DOI: 10.1016/j.ccell.2025.09.003