In the war between viruses and humans, there’s no battlefield more intimate than the inside of a living cell. Every moment, invisible invaders infiltrate and hijack the machinery of life, turning human biology into their personal production factory. But now, a new study has exposed a shocking tactic used by one of humanity’s most familiar foes: the herpes simplex virus-1 (HSV-1), known best as the culprit behind cold sores.
In a paper published in Nature Communications, scientists at the Center for Genomic Regulation (CRG) in Barcelona reveal that HSV-1 doesn’t merely hijack a cell’s tools—it completely remodels the genetic landscape to suit its needs. With surgical precision, it reconfigures the human genome’s 3D structure, unlocking access to the genes it needs most to replicate.
It’s not just a viral infection. It’s an interior redesign of our DNA.
An Intruder with a Blueprint
“HSV-1 is an opportunistic interior designer, reshaping the human genome with great precision and choosing which bits it comes into contact with,” said Dr. Esther González Almela, the study’s first author. “It’s a novel mechanism of manipulation we didn’t know the virus had.”
The discovery upends decades of understanding about how viruses interact with the host genome. Until now, scientists believed that any genome distortion caused by viruses was simply collateral damage—unintended structural consequences of viral invasion. But this new research reveals that HSV-1’s genome manipulation is anything but accidental.
Within just an hour of infection, HSV-1 launches a stealthy reorganization of the host cell’s DNA, changing which sections are exposed and which are buried—redecorating the genomic architecture to grant the virus front-row access to the genes that fuel its replication.
A Hijacking in High Resolution
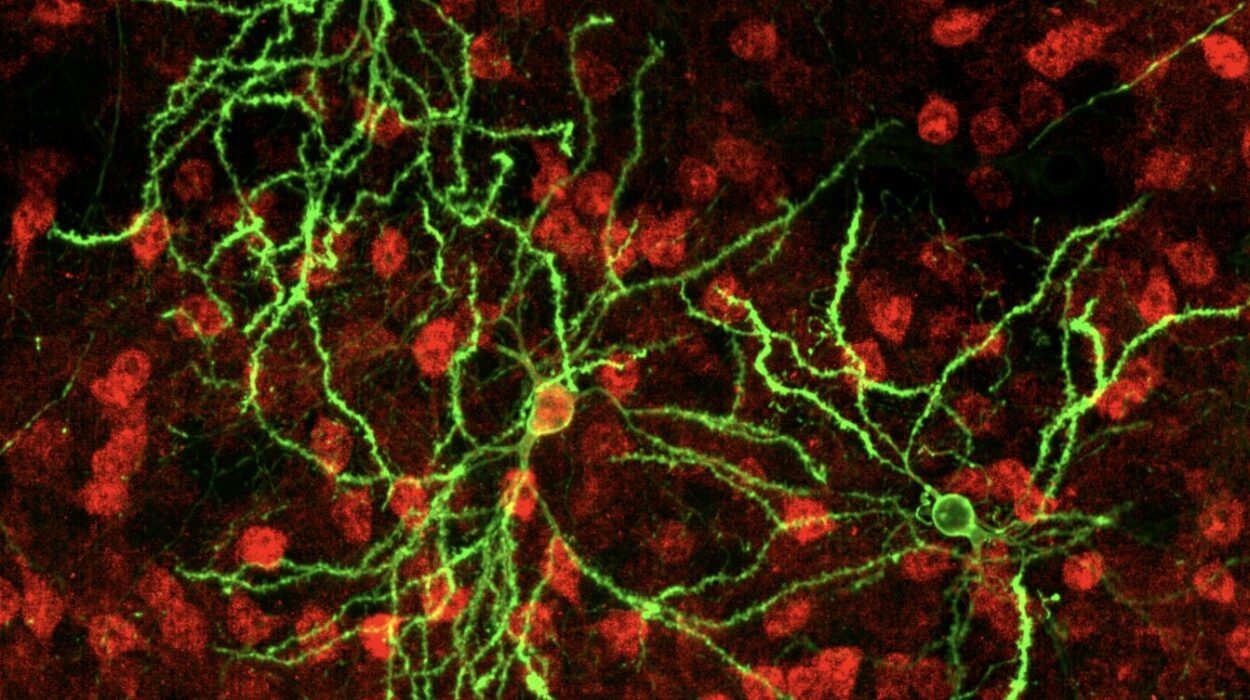
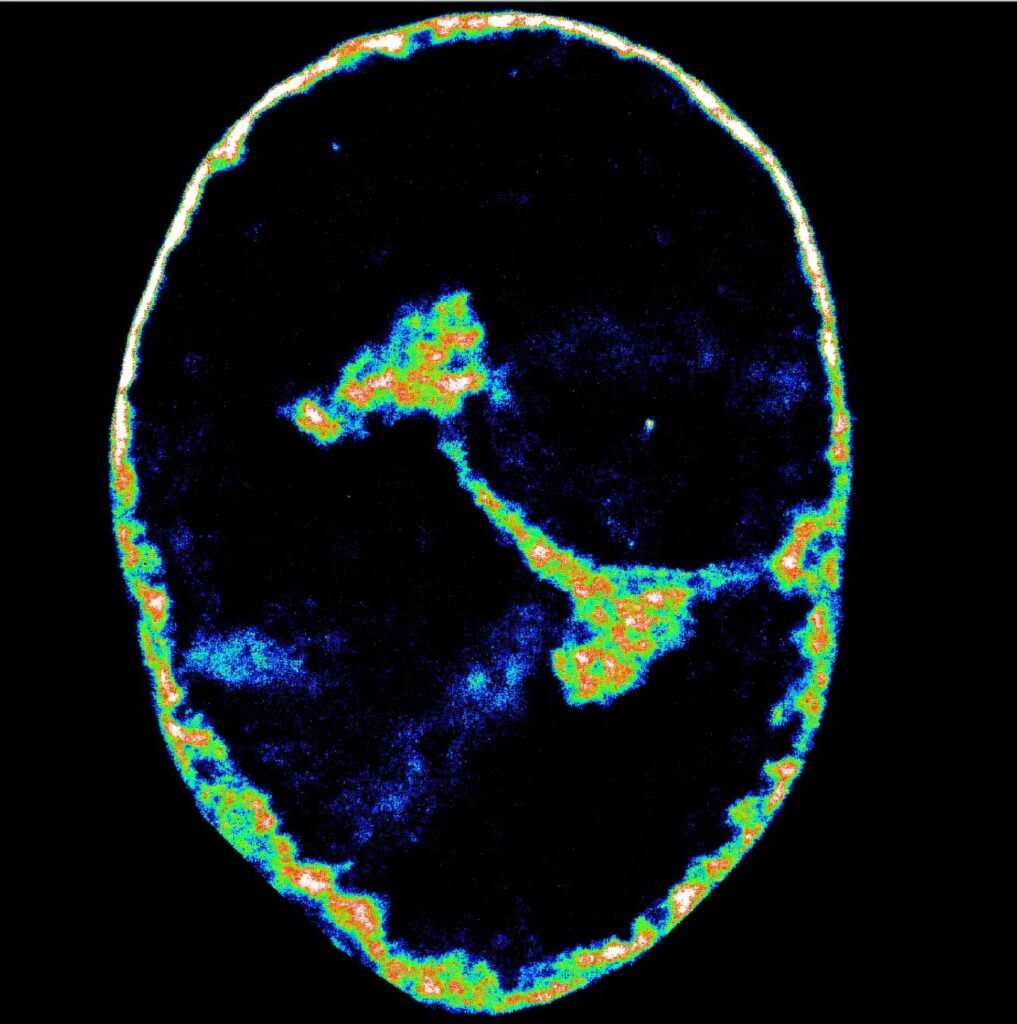
To unravel this microscopic coup, researchers turned to cutting-edge technology. Using super-resolution microscopy, capable of resolving structures 3,500 times thinner than a human hair, they peered deep into the cell’s nucleus. Alongside this, they used Hi-C, a genomic mapping tool that reveals which regions of DNA are physically close inside the nucleus—a sort of molecular census of the genome’s social network.
With these tools, the team captured HSV-1’s assault in real-time.
In the earliest stages of infection, the virus hijacks the host’s RNA polymerase II—the enzyme that normally helps cells read their own DNA and make proteins. HSV-1 lures it into newly formed viral replication compartments, like an intruder who tricks the workers of a factory into building weapons for the enemy.
Once the polymerase is inside, other crucial players follow: topoisomerase I, an enzyme that snips DNA to relieve stress during transcription, and cohesin, a structural protein that maintains the genome’s shape. These, too, are redirected away from their regular posts and repurposed for the virus’s goals.
The Collapse of Cellular Control
By the three-hour mark, the cell is no longer in charge of its own instructions.
Most of the RNA polymerase II has vacated the human genome, and with it, topoisomerase I and cohesin. The host’s transcription machinery—responsible for producing all the proteins that keep the cell alive—begins to collapse. Genes fall silent. Messages stop being sent. It’s as if an entire newsroom has been abruptly abandoned mid-broadcast.
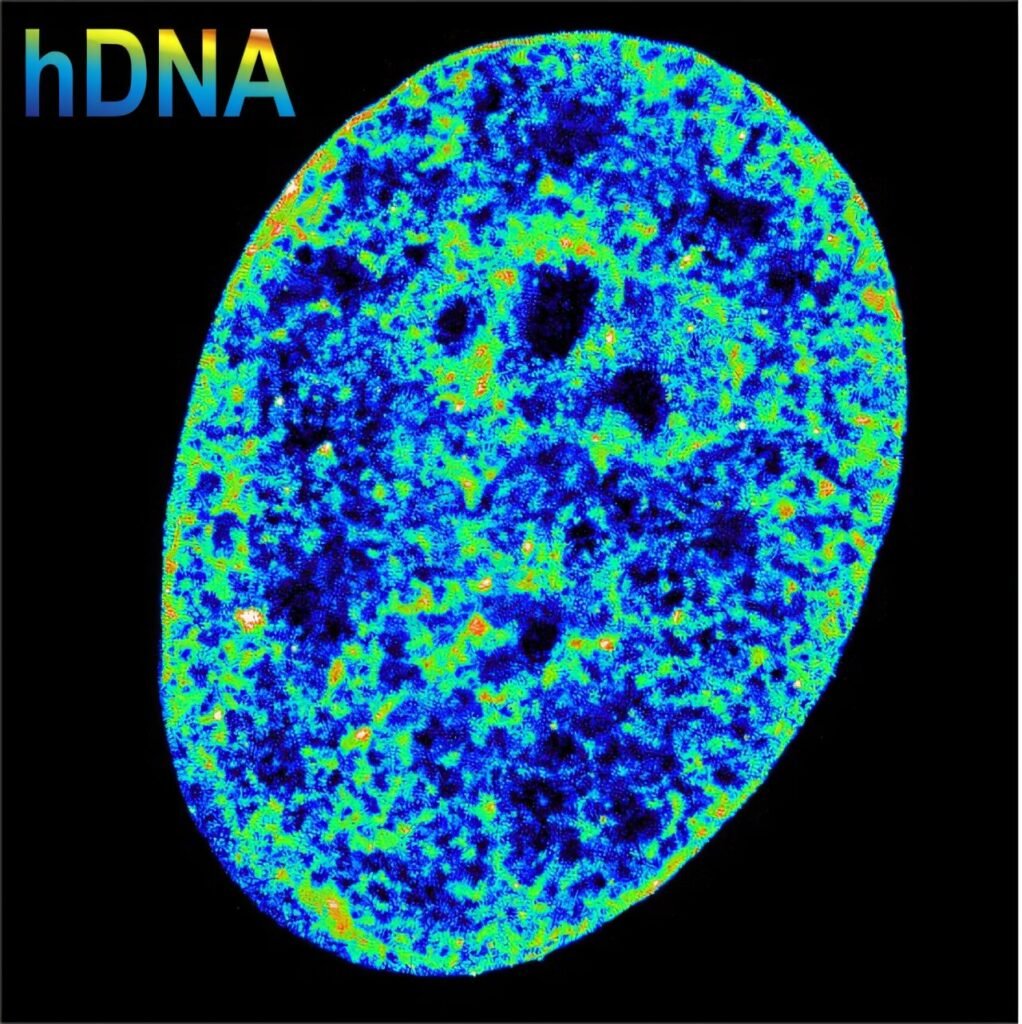
Then, something startling happens.
The researchers observed that the chromatin, the natural package of DNA in the nucleus, began to shrink. Normally, chromatin’s structure determines which genes are active. Looser packaging allows genes to be expressed, while tighter packaging usually locks them down. But here, the researchers found the reverse.
“We always thought dense chromatin shut genes down, but here we see the opposite: stop enough transcription first, and the DNA compacts afterwards,” said Dr. Álvaro Castells García, co-first author of the study. “The relationship between activity and structure might be a two-way street.”
It’s a fundamental shift in how we understand the mechanics of gene regulation. The virus doesn’t just disrupt gene activity—it reorders the DNA itself, and the cell’s genome responds by collapsing in on itself, shrinking to just 30% of its original volume.
Stopping the Virus in Its Tracks
One of the most promising revelations came not just in observing what HSV-1 does—but in learning how to stop it.
The researchers discovered that blocking a single enzyme—topoisomerase I—brought the entire viral operation to a halt. Without it, HSV-1 could not rearrange the genome. It couldn’t build replication centers. It couldn’t reproduce.
“In cell culture, inhibiting this enzyme stopped the infection before the virus could make a single new particle,” explained ICREA Research Professor Pia Cosma, the study’s senior author. “That gives us a potential new therapeutic target to stop infection.”
It’s an enticing possibility in the world of virology: a weapon to halt HSV-1 at the door, before it can build a single strand of its own DNA.
An Ancient Virus with a Global Grip
Herpes simplex virus-1 is no stranger to the human species. By some estimates, two out of every three people under age 50 carry the virus. Most never show symptoms. Others live with recurring cold sores, flare-ups triggered by stress or illness.
But HSV-1 isn’t always benign. In rare cases, it can cause blindness, encephalitis, or life-threatening disease in newborns and the immunocompromised. It is one of the most common and persistent infections on Earth, and yet we have no cure.
Antiviral medications like acyclovir can manage symptoms, but drug-resistant strains are increasing, and the virus, once caught, hides in the nervous system forever.
That’s what makes this study so compelling. By revealing the virus’s step-by-step method of rewriting the genome—and identifying a chokepoint in the process—researchers have opened a door to something that once seemed out of reach: stopping HSV-1 in its tracks.
A New Way to Understand Infection
More than just a virological breakthrough, the study hints at a new paradigm in biology. The way a virus reshapes the genome, not only to suppress host defense but to actively select which parts of the genome to exploit, paints viruses not just as invaders but as molecular engineers.
The implications extend beyond HSV-1. If other viruses use similar mechanisms to rewire host DNA architecture, there may be untapped opportunities to intervene, slow, or block viral replication through structural interference.
It also reframes how scientists might approach diseases where chromatin architecture and transcription are disrupted—including cancers and autoimmune conditions. Could viruses be teaching us new ways the genome can be restructured—and how cells can recover from such manipulation?
The Future of Fighting Viruses
The road ahead is long. The enzyme topoisomerase I is essential to all cells, not just to viruses. Any therapeutic would need to be precisely targeted, perhaps with a window short enough to stop infection but gentle enough to avoid harming the host.
Still, the blueprint is there.
This study delivers more than just insight into how a common virus operates. It’s a reminder that the battlefield of infection isn’t just about the immune system or antibodies. Sometimes, it’s about architecture, about structure, about who controls the blueprint of life at the molecular level.
In HSV-1’s case, the virus arrived with its own design—and for the first time, scientists have caught it red-handed, blueprint in hand, mid-remodel.
And now, perhaps, we know how to lock the door.
Reference: Herpes simplex virus type 1 reshapes host chromatin architecture via transcription machinery hijacking, Nature Communications (2025). DOI: 10.1038/s41467-025-60534-6