In a brightly lit laboratory tucked inside the Life Sciences Institute at the University of Michigan, something remarkable just happened. A team of biochemists has cracked open one of HIV’s long-held secrets—a sleight of hand it uses to commandeer the tiny molecular highways inside our cells. In doing so, they’ve not only overturned a decades-old assumption in virology, but they’ve also unlocked a new window into how viruses stealthily navigate our cells, potentially revealing new targets for future antiviral drugs.
This isn’t just another virus study. It’s a shift in how we understand the fundamental mechanics of viral infection, thanks to a simple but revolutionary idea: what if we could watch HIV move—without the chaos of a living cell getting in the way?
How HIV Gets Around Inside Us
To grasp the significance of this discovery, imagine the inside of a cell not as a chaotic soup, but as an intricately choreographed city. Inside every living cell, thousands of tiny compartments and proteins are in constant motion, carried from one place to another along an internal road system made of microtubules—threadlike structures that act like highways. The cargo—everything from energy-producing mitochondria to virus particles—is ferried by microscopic machines called motor proteins.
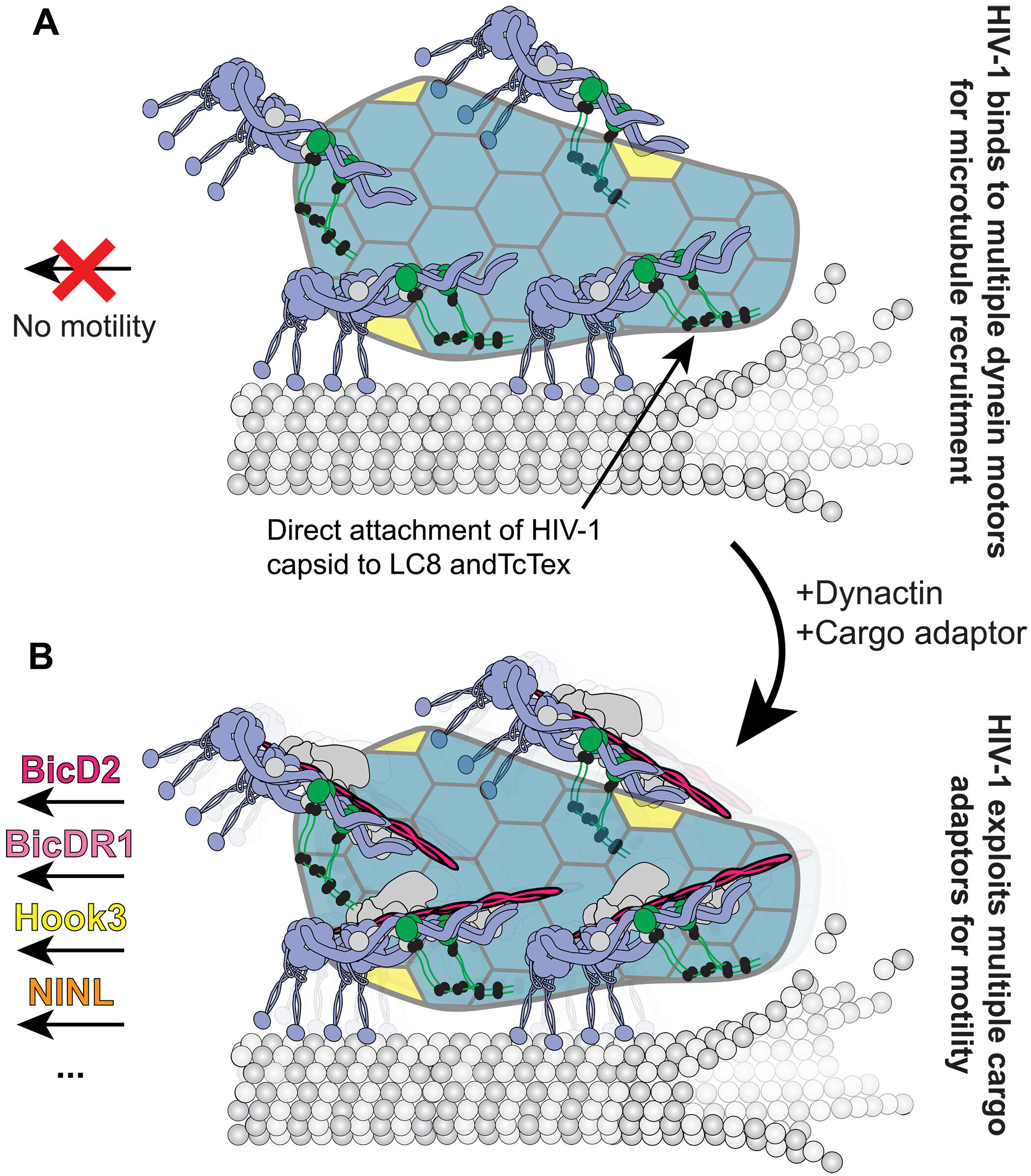
One of the most important of these molecular machines is dynein. It’s a cellular workhorse, tirelessly carrying its cargo from the outer edges of the cell toward the nucleus, where the cell’s genetic blueprint is stored. And HIV, the virus that causes AIDS, has long been known to exploit dynein to smuggle its own genetic material into the host cell’s core, a key step in its replication and infection process.
Until now, scientists believed that HIV needed a very specific helper to do this: a cargo adapter protein named BicD2. Think of BicD2 as a kind of trailer hitch, hooking the virus to the dynein truck and enabling it to move. This was the accepted view since the early 2000s—a tidy model that made sense, but one that, as it turns out, wasn’t quite true.
Watching the Virus Walk
The new research, published this week in Science Advances, reveals that HIV is far more adaptable and opportunistic than anyone thought. Led by Dr. Michael Cianfrocco, a biochemist at the University of Michigan’s Life Sciences Institute and Medical School, the study used a bold and novel approach: watching HIV move, outside of a living cell.
“To understand exactly how HIV traffics within cells, we needed to simplify the system,” said Cianfrocco. “Inside a cell, everything’s noisy—there are so many interactions happening at once. We wanted to isolate the players and watch them in action.”
So the team did something extraordinary. They purified individual proteins—the dynein motor, accessory proteins, and parts of the virus itself—and reconstructed the transport process on a glass microscope slide. Using high-resolution imaging, they could literally watch dynein motors ‘walk’ along microtubule highways, dragging viral capsids—HIV’s genetic containers—behind them.
This stripped-down system offered clarity that living cells, with all their complexity, simply couldn’t provide.
An Unexpected Discovery
What they saw overturned two decades of accepted wisdom.
HIV didn’t need BicD2 at all.
Instead, the virus latched directly onto the dynein motor—no trailer hitch required. It was only when it needed the motor to start moving that another adapter protein had to get involved. But—and this is the critical twist—it could be any adapter protein, not just BicD2.
“This flexibility means HIV can take advantage of whatever’s available in the cell it’s infecting,” explained Dr. Somaye Badieyan, lead author of the study and a research scientist in Cianfrocco’s lab. “It doesn’t have to wait for one specific adapter protein. It can hijack any one that’s handy and catch a ride to the nucleus.”
In other words, HIV is a far more cunning passenger than previously believed. It doesn’t just wait at the curb for its preferred driver—it flags down whatever taxi happens to be passing by.
Why This Changes Everything
The implications of this are huge. For years, researchers were looking at HIV’s trafficking with blinders on, focusing on BicD2 as the essential component. But this study shows the virus is much more versatile, able to hijack a wider variety of cellular machinery than anyone knew.
This not only changes our understanding of how HIV infects cells, but it also broadens the scope for potential drug development. If the virus can use multiple adapter proteins to move, then blocking just one—like BicD2—might not be enough. But now, with a new system in hand, researchers can start to identify other potential weak points in the viral infection process.
“This study gives us a whole new platform,” said Cianfrocco. “Now that we know how to recreate viral trafficking with purified components, we can add different variables one at a time. We can test how the virus behaves with different adapters, or how mutations affect movement, or how new drugs might interfere.”
That’s never been possible before. Most virology experiments take place inside living cells, where countless processes compete for attention. But this minimalist approach strips away the noise and focuses the lens on the mechanics of viral motion.
A First in Viral Trafficking Research
The study marks the first time that scientists have successfully recreated viral transport using purified components outside the cell. That might sound niche, but it’s a landmark breakthrough. Viruses, by their very nature, can’t survive on their own. They need host cells to do everything—from replicating genetic material to building new virus particles. That’s made them incredibly difficult to study in isolation.
But now, thanks to this reconstitution system, the pieces can be taken apart and examined like never before. Researchers can start to treat viral infection like a modular puzzle, identifying not just how HIV gets in, but how it moves, where it stalls, what powers it, and how to stop it in its tracks.
“This study introduces a completely new way to think about viral infection,” said Badieyan. “It’s not just about preventing entry anymore. It’s about blocking movement, too—finding ways to derail the virus before it reaches its destination.”
Toward a New Era in Antiviral Strategy
Though this discovery doesn’t offer an immediate cure for HIV, it’s an essential step in the long game of understanding—and outsmarting—the virus. With roughly 39 million people living with HIV worldwide, according to UNAIDS, even small advancements in our understanding of its biology carry enormous weight.
The adaptability shown by HIV in this study also raises important questions about other viruses. Could influenza, herpes, or even SARS-CoV-2 use similar mechanisms to hijack cell transportation systems? Could their movement be disrupted using similar experimental tools? Cianfrocco’s team believes their approach could easily be expanded to explore these questions.
What’s more, the reconstitution system may even help researchers design synthetic viral particles for gene therapy—safe versions of viruses that can be programmed to deliver helpful genetic material to specific places inside the cell.
A Microscopic Ballet, Finally Understood
As the purified proteins danced across the glass slide under a microscope—dynein motors stepping along microtubules, viral capsids in tow—the researchers at Michigan were witnessing a kind of molecular ballet that had long been hidden from view. For the first time, they had isolated the choreography of infection, frame by frame.
And what they saw wasn’t just science—it was strategy. A virus, tiny and lifeless on its own, outwitting human cells through flexibility, resilience, and opportunism.
HIV, it seems, isn’t just a passenger. It’s a master of improvisation. And now, at last, we’re learning how to see its movements clearly enough to stop them.
Reference: Somayesadat Badieyan et al, HIV-1 binds dynein directly to hijack microtubule transport machinery, Science Advances (2025). DOI: 10.1126/sciadv.adn6796