Of all the human body’s organs, the liver holds a singular distinction: it can regenerate itself. This isn’t just folklore or medical myth—it’s a biological fact. Unlike the heart or brain, which cannot repair themselves after significant damage, the liver has an almost mythic ability to bounce back. Remove up to two-thirds of it, and within days, the remaining portion will start growing, repopulating its structure, and restoring function. It is as if this organ remembers its original form and is determined to reassemble itself.
But how does this happen? What signals tell liver cells to divide, differentiate, and take up the burden of full metabolic function again? For decades, the mechanisms behind liver regeneration remained elusive, their clues buried deep in the genome’s silent scaffolding. Now, a groundbreaking study from the University of Barcelona, recently published in Cell Genomics, reveals that the answers may lie not in the genes themselves, but in the regulatory regions of DNA—the hidden “switches” that control when and how genes are activated.
This new research has not only pinpointed the genetic circuits that turn on liver regeneration—it’s begun to chart a map of the genome’s command center, offering insights that could one day transform regenerative medicine.
The Molecular Orchestra Behind a Regrowing Organ
Led by Palmira Llorens-Giralt and professors Florenci Serras and Montserrat Corominas from the University of Barcelona’s Department of Genetics, Microbiology and Statistics, this collaborative effort brought together scientists from some of Spain’s most prestigious research institutions. Using mouse models, they conducted a sophisticated exploration of chromatin—the complex, dynamic packaging of DNA that determines whether genes are accessible or silenced at any given time.
Rather than looking at which genes were turned on, they looked at how they were turned on. This distinction is vital. Genes are the machinery, but enhancers—the regulatory sequences of DNA that do not code for proteins—are the technicians controlling the switches. The genome is not merely a book of instructions; it’s a musical score, and enhancers are the conductors of a molecular symphony.
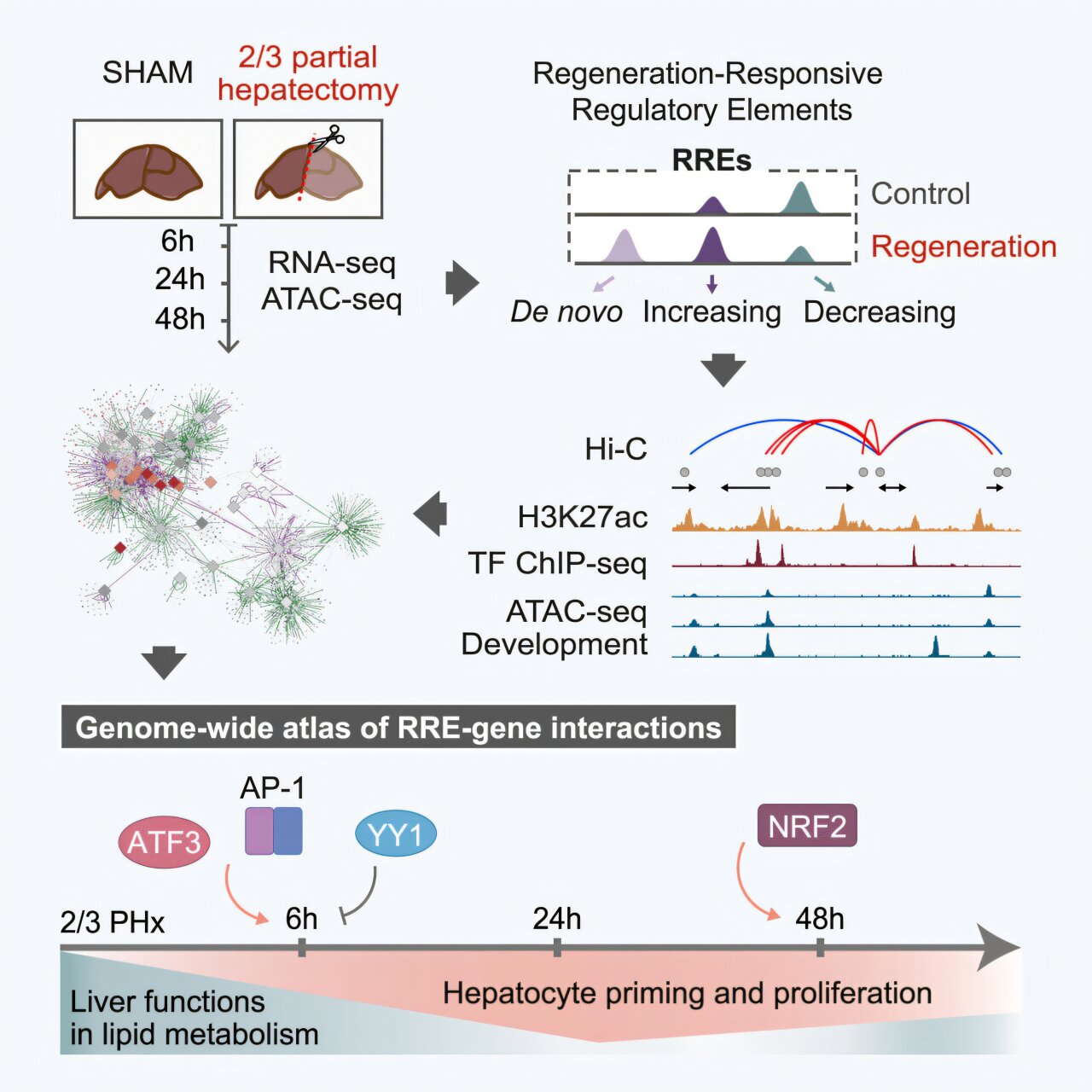
Their work focused on what happens after partial hepatectomy, a procedure where a significant portion of the liver is surgically removed. Common in cancer treatment and living donor transplants, this operation forces the liver to jumpstart its regenerative engine. It’s during this regeneration that the researchers captured a dynamic, genome-wide snapshot of enhancer activity, transcriptional shifts, and chromatin remodeling.
Their findings provide a revolutionary look into the genomic choreography that allows the liver to rebuild itself—by reactivating not just generic growth programs, but some of the very same DNA switches used during embryonic development.
When Development Repeats Itself
One of the study’s most compelling discoveries was that liver regeneration mimics, in many respects, the liver’s original development in the embryo. Through careful chromatin mapping and transcriptional profiling, the researchers observed that regeneration draws upon two distinct types of enhancers.
Some are unique to the regenerative process: they are activated only in response to injury, like emergency lights switched on in a blackout. But intriguingly, others are reactivated from earlier stages of life—specifically, enhancers that had been active during embryonic liver development. These developmental enhancers are normally dormant in adults, tucked away like blueprints in a dusty archive. Yet when the organ is injured, the liver seems to dig up these old schematics, retracing the developmental steps it once took in utero.
This reactivation is not simply a duplication of embryonic growth; it’s a refined, context-sensitive program. Developmental enhancers are turned back on in adult cells, not to create a new organ from scratch, but to fuel the expansion and repatterning of the existing one. It’s an elegant recycling of genetic history, repurposed to meet a present crisis.
Trading Metabolism for Mitosis
Regeneration comes at a metabolic price. One of the more counterintuitive revelations from the study is that, during regeneration, the liver temporarily suppresses some of its primary functions—especially those related to fat and lipid metabolism.
This strategic downregulation is orchestrated through the silencing of specific enhancers associated with metabolic pathways. Enhancers responsible for regulating bile acid production, cholesterol synthesis, and vitamin A metabolism are effectively shut down during the early stages of regrowth. In their place, a different set of enhancers—those that promote cellular proliferation—take center stage.
What emerges is a kind of biological triage. The liver doesn’t attempt to do everything at once. Instead, it prioritizes regrowth over routine function. Much like a city after an earthquake, the liver diverts its resources from day-to-day operations to critical infrastructure repair. Once structural and cellular integrity are restored, the metabolic machinery gradually powers back up.
This elegant switch from function to regeneration and back again underscores how deeply regulated this process is—and how finely tuned the balance between life-sustaining function and cellular renewal must be.
The Master Switches: Transcription Factors in Command
Genes do not operate in a vacuum. Their activity is governed by transcription factors—proteins that bind to enhancers and other regulatory elements to either activate or suppress gene expression. The study identified several key transcription factors that act as molecular gatekeepers during the liver’s regenerative response.
In the immediate aftermath of resection, two main players take the stage: AP-1 (Activator Protein 1) and ATF3 (Activating Transcription Factor 3). These proteins are known for their roles in responding to stress and injury in a wide array of tissues. In the liver, they appear to function as first responders, binding to regeneration-specific enhancers and launching the transcriptional programs that drive hepatocytes—normally dormant—into rapid proliferation.
Once the initial wave of division is underway, a second transcription factor, NRF2 (Nuclear Factor Erythroid 2–Related Factor 2), takes over. Known for its role in oxidative stress response, NRF2 also appears to guide the maintenance phase of regeneration, sustaining the proliferative momentum and possibly coordinating the handoff back to metabolic function.
These insights highlight a temporal layering of regulation. The liver doesn’t just regenerate—it does so in a tightly choreographed sequence of stages, each governed by different sets of molecular actors. Understanding this cascade opens new avenues for intervention: drugs or gene therapies could one day mimic or enhance the effects of these transcription factors to accelerate healing or support failing livers.
Chromatin: The Shape of Recovery
At the heart of these discoveries is chromatin—the complex of DNA and protein that makes up chromosomes. Chromatin isn’t just packaging; it’s an active participant in gene regulation. Its structure determines which parts of the genome are accessible and which are hidden, playing a central role in enhancer activity.
The study used chromatin immunoprecipitation and high-throughput sequencing techniques to map these structural changes. They observed that regions of chromatin associated with regenerative enhancers opened up dramatically post-hepatectomy, making them accessible to transcription factors. Conversely, chromatin regions linked to metabolic enhancers became more tightly packed and less accessible, correlating with their silencing.
This shifting chromatin landscape resembles a molecular theater set—where spotlights illuminate certain areas of the stage while others fade into darkness. The accessibility of enhancers isn’t fixed; it’s dynamic, controlled, and crucial to the script of regeneration.
Implications Beyond the Mouse Model
Though conducted in mice, the study’s findings resonate far beyond the animal model. Human livers operate under the same basic principles of cellular biology, and many of the enhancers and transcription factors identified have homologs in the human genome. This research is foundational—it doesn’t promise a pill tomorrow, but it opens the door to a host of future innovations.
One possible application is in the field of regenerative medicine. If we can identify drugs or genetic therapies that activate the same enhancers or mimic the effects of AP-1, ATF3, and NRF2, we could potentially stimulate liver regeneration in patients with chronic liver diseases, or optimize liver recovery after surgery. This could be life-changing in conditions like cirrhosis, hepatitis, or fatty liver disease, where regeneration is often impaired or insufficient.
Another tantalizing avenue is bioengineering. If scientists can guide stem cells or tissue-engineered organs to follow the same enhancer activation pattern, we might one day grow liver tissue in the lab that is functionally indistinguishable from native organs—right down to its regenerative capabilities.
A Blueprint for Future Breakthroughs
Perhaps the most important contribution of this research is the genome-wide map it provides—a detailed atlas of enhancer-gene interactions during liver regeneration. This resource is now available for the broader scientific community and offers a starting point for countless new studies.
With this map, researchers can test which enhancers are conserved across species, which are specific to certain stages of regeneration, and how they interact with various epigenetic factors. It also allows for the modeling of regenerative pathways in silico, where computational biology can simulate the impact of drugs, mutations, or transcriptional changes on liver recovery.
The value of such a map cannot be overstated. It is the Rosetta Stone for understanding not only liver regeneration but potentially other forms of tissue regeneration where enhancers play a central role. Similar strategies could be employed to decode the regenerative capabilities of skin, muscle, or even neural tissue.
The Path Ahead: From Basic Biology to Bedside Care
The researchers involved are quick to note that this is still basic science. The goal of the study was not to develop a treatment, but to understand the biology of regeneration at its most fundamental level. Yet it’s precisely this kind of foundational work that forms the bedrock of medical progress.
Breakthroughs in medicine rarely arrive in a single leap. They are built incrementally, often from studies like this—deep, detailed investigations that peel back the layers of nature’s machinery to reveal how it works. And once the mechanisms are understood, interventions become possible.
The prospect of translating these findings into human therapies is a long-term endeavor. Still, the groundwork has been laid. Scientists now have the molecular keys to the liver’s regenerative code, and with them, a better chance of unlocking treatments for some of the most challenging conditions in modern medicine.
Regeneration, Reimagined
The story of liver regeneration is, in many ways, a story of resilience. It’s the tale of how cells remember their origins, how genetic blueprints from early life can be summoned in crisis, and how a single organ has evolved a near-miraculous capacity to repair itself.
With the insights provided by this research, we are beginning to move from admiring that resilience to understanding it—and ultimately, to harnessing it. Whether through drugs that activate specific enhancers or gene therapies that mimic developmental programs, the future of regenerative medicine is now rooted more deeply in the genome than ever before.
The liver, long a symbol of vitality and rebirth in ancient myth, is once again proving to be one of the most extraordinary tissues in the body. And thanks to the efforts of researchers mapping its hidden regulatory circuitry, we may be entering an era where regeneration is no longer a miracle of nature, but a science within our grasp.
Reference: Palmira Llorens-Giralt et al, Sequential activation of transcription factors promotes liver regeneration through specific and developmental enhancers, Cell Genomics (2025). DOI: 10.1016/j.xgen.2025.100887