Every breath we take is an unconscious miracle—until it’s not. When lungs are injured by trauma, infection, or toxic exposure, they fill not just with pain but with fluid. What should be an open, airy exchange of gases becomes a heavy, suffocating struggle. In these moments, the body mobilizes a silent army: the lymphatic system, an often-overlooked network tasked with draining excess fluid, ferrying immune cells, and restoring balance. But how this system adapts and responds in the chaos of lung injury has long remained mysterious.
Now, in a groundbreaking study published July 17 in Nature Cardiovascular Research, scientists from Weill Cornell Medicine have illuminated a key player in this process. A protein called PAR1 acts like a molecular switch, allowing the lymphatic vessels in the lungs to physically reshape themselves in response to injury. This shape-shifting isn’t cosmetic—it enables the vessels to absorb more fluid and immune cells, accelerating healing and protecting lung function in the face of damage.
Unlike blood vessels, where permeability often signals leakage and disease, the transformation triggered by PAR1 in lymphatic vessels is therapeutic. It’s a reminder that the body’s repair systems aren’t passive—they’re adaptable, even architectural.
A Doorway That Opens on Demand
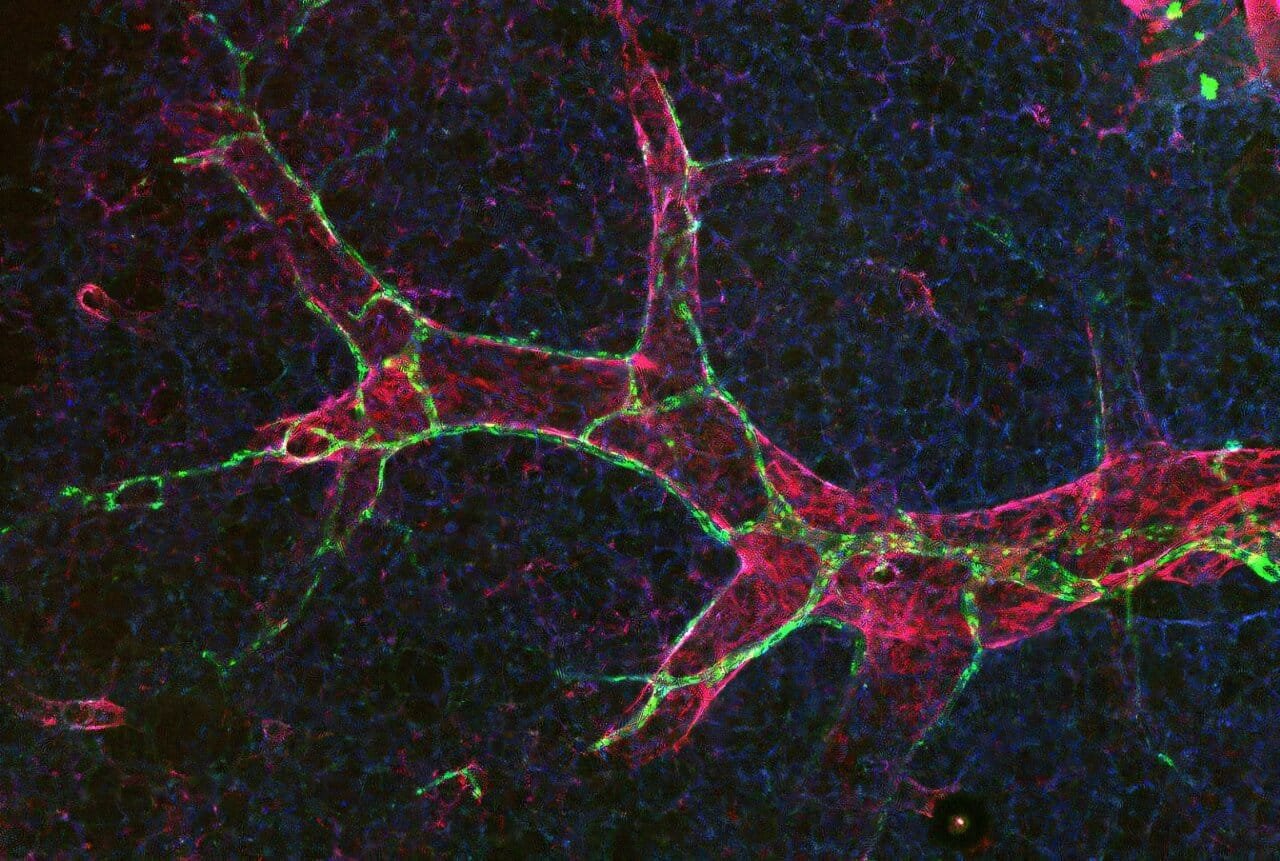
Lymphatic vessels are built with doors. Not literal ones, but specialized junctions between the endothelial cells lining the inner walls of these vessels. These cell junctions determine what gets in, what stays out, and how efficiently the system can respond during times of crisis. The two most prominent types of junctions are metaphorically described as buttons and zippers.
Dr. Hasina Outtz Reed, assistant professor of pulmonary and critical care medicine at Weill Cornell Medicine and the study’s principal investigator, explains the distinction with intuitive imagery: “Button junctions are like the buttons on a shirt—you can slip fingers through the gaps between them. Zipper junctions, on the other hand, are tightly sealed, like the teeth of a zippered hoodie.”
In healthy lungs, a surprising number of lymphatic junctions are zipped shut—limiting permeability. But when injury strikes, that equation changes dramatically. The team’s mouse model experiments revealed that injury triggers a rapid remodeling of these junctions: zippers unzip into buttons, turning tightly closed barriers into absorptive gateways. This transformation allows fluid to drain, immune cells to enter, and the healing process to begin.
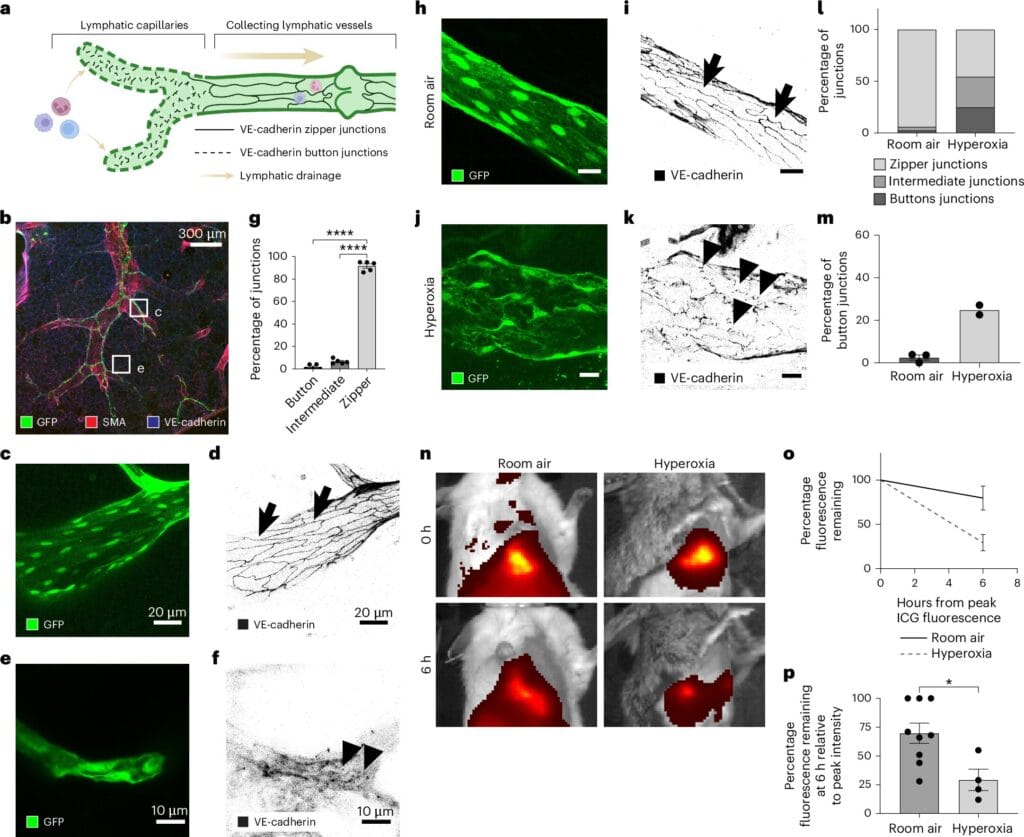
Crucially, this shift doesn’t occur in blood vessels—where increased permeability often contributes to disease. This unique response in lymphatic vessels hints at a deeply specialized and protective function, fine-tuned over millennia of evolution.
PAR1: The Master Key to the Lymphatic Door
At the heart of this biological choreography is PAR1, short for protease-activated receptor 1. While PAR1 has been studied in cardiovascular disease and cancer for its role in coagulation and vascular remodeling, its significance in the lymphatic system has only recently come to light.
Dr. Outtz Reed’s team discovered that PAR1 is essential for the zipper-to-button transition in lymphatic endothelial cells. When mice were genetically engineered to lack PAR1, their lymphatic vessels remained zipped shut—even in the face of acute lung inflammation. The result was predictable and perilous: fluid pooled in the lungs, immune cells were stranded, and the healing process was severely impaired.
Without PAR1, the vessels couldn’t adapt. They remained sealed when they needed to open. In effect, the lungs were left to drown in their own defenses.
This finding opens a new biological frontier. PAR1 isn’t just a receptor; it’s a structural architect. It tells the cells of the lymphatic system when to loosen up, when to embrace permeability, and when to return to a sealed state once the crisis has passed. It’s not just responsive—it’s intelligent.
The Quiet Genius of the Lymphatic System
The lymphatic system is often described as the body’s “second circulatory system,” but it’s much more than that. In the lungs, it doesn’t just drain excess fluid—it also transports immune cells, manages inflammation, and maintains a delicate internal balance known as homeostasis. It’s a regulatory system, a sanitation service, and an immune highway rolled into one.
Yet for most of medical history, the lymphatics—particularly in the lungs—were neglected. As Dr. Outtz Reed puts it, “Despite their critical role, lymphatics have been historically overlooked until recently, and lung lymphatics have been particularly understudied.”
That’s beginning to change. The new research reveals not just their importance, but their elegance. The idea that these vessels can physically remodel themselves in response to injury—modifying cell junctions in real time—is a testament to the system’s precision and flexibility.
This fluidity may be especially important in the lungs, where space is limited and timing is everything. A buildup of fluid can quickly become fatal. An overzealous immune response can cause more damage than the injury itself. The lymphatic system must walk a tightrope between drainage and restraint. PAR1 helps it stay balanced.
A Cautionary Tale for Drug Development
The discovery also comes with a warning. PAR1 has been a target in numerous drug trials, especially in cardiovascular medicine, where blocking its function was believed to prevent clot formation and reduce vascular disease. But many of these trials have failed—or yielded unexpected side effects.
The Weill Cornell team now believes that at least part of the explanation lies in the dual nature of PAR1. In blood vessels, blocking PAR1 might be beneficial. But in lymphatic vessels—especially those in the lungs—PAR1 activity appears to be protective. Shut it down entirely, and you may unintentionally paralyze the body’s fluid drainage system.
“A lot of clinical trials targeting PAR1 have been unsuccessful,” says Dr. Outtz Reed. “We think, in part, this may be due to the lymphatic vasculature, which also expresses this receptor, but responds to the drug in completely different ways than intended.”
This means that future therapies need to be more nuanced. It’s no longer enough to think of the body as one unified field where a single drug affects all tissues the same way. Instead, precision medicine must consider where a receptor is expressed, how it behaves in that context, and what side of the healing equation it sits on.
A New Frontier in Lung Healing
For co-author Dr. Chou Chou, the journey to this discovery was deeply personal. “Seeing countless patients with severe lung injury as a medical resident five years ago made me wonder why we still don’t have targeted therapies for them,” he said. “We hope this research gives us a more complete picture of the lungs during severe injury and opens new avenues for therapies.”
Such therapies could one day selectively activate PAR1 in lung lymphatics—promoting drainage and immune regulation—without affecting blood vessels or other tissues. It’s a delicate goal, but a critical one. In conditions like acute respiratory distress syndrome (ARDS), pneumonia, or even severe COVID-19, the body’s inability to clear fluid and regulate inflammation can be deadly.
Future research will explore how these mechanisms operate in the presence of pathogens—how viral or bacterial invaders may disrupt, hijack, or exploit the zipper-to-button transition. Dr. Outtz Reed’s lab is already working on how to selectively modulate PAR1 in lymphatics while leaving blood vessels untouched.
The Hope Beneath the Surface
In the end, this study is a story about seeing what’s hidden. About looking beyond the obvious—blood vessels, visible injury, major organs—and finding brilliance in the background systems that quietly keep us alive. It’s a story of molecular empathy: of how cells respond not just with panic, but with strategy and elegance.
The discovery of PAR1’s role in lung lymphatic remodeling does more than fill a scientific gap. It challenges the long-held belief that the body’s response to injury is blunt and chaotic. Instead, it offers a glimpse into a healing process that is dynamic, architectural, and deeply intelligent.
For the millions of patients around the world who suffer from inflammatory lung conditions—and for the clinicians who care for them—this discovery could mark the beginning of a new era. An era where we don’t just treat symptoms, but partner with the body’s own adaptive machinery to restore balance, breath, and life.
Reference: The thrombin receptor PAR1 orchestrates changes in lymphatic endothelial cell junction morphology to augment lymphatic drainage during lung injury, Nature Cardiovascular Research (2025). DOI: 10.1038/s44161-025-00681-7, www.nature.com/articles/s44161-025-00681-7