After a sleepless night, there’s a particular kind of drowsiness that creeps into the body—a thick, almost gravitational pull toward rest. For many, this ends in a late-afternoon nap or an extended slumber the next night, the body making up for lost hours. Scientists call this process sleep homeostasis—the way our internal systems keep track of sleep debt and demand repayment.
Now, in a breakthrough that could change the way we understand and treat disrupted sleep, researchers have identified a specific group of neurons that may hold the keys to this restorative balancing act. The discovery, made in mice, shines a spotlight on a part of the brain called the thalamus, where a set of excitatory neurons named REVglut2 orchestrates the descent into deep sleep after prolonged wakefulness.
Published in the journal Science, the study offers a major step toward understanding how the brain physically builds the need for sleep—and how it eventually pays the bill.
The Thalamic Timekeepers
Sleep is a biological necessity as universal as breathing. From humans to flies, nearly every animal rests. Yet the question of how the brain knows when we’re tired—and how it triggers recovery after deprivation—has long puzzled neuroscientists. While the discovery of wake-promoting neurons in the 1940s opened up the study of arousal and alertness, the sleep side of the equation remained murkier.
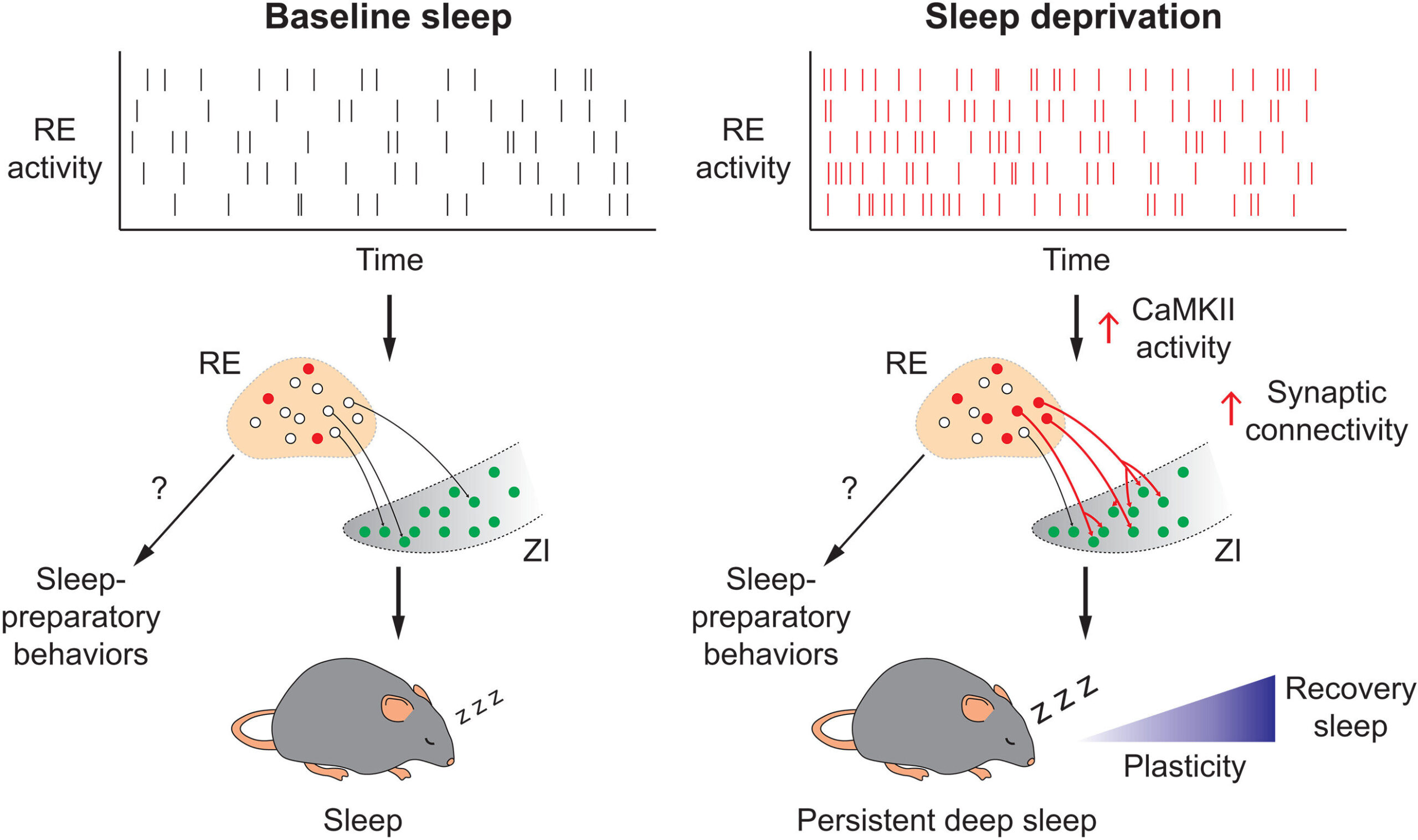
The new research zeroes in on a thalamic region called the nucleus reuniens (RE)—a deep-seated brain area previously associated with attention and memory. There, scientists uncovered a population of excitatory neurons—REVglut2—that act as a kind of internal sleep sensor. These neurons don’t just passively record fatigue—they actively respond to it.
Using a method known as retrograde viral tracing, the research team mapped how these neurons interact with other sleep-related brain regions. The tracing technique allowed scientists to follow the paths of communication, showing that the REVglut2 neurons reach out to various parts of the brain known to promote non-REM (NREM) sleep—the slow, deep, and most restorative phase of slumber.
Perhaps most intriguingly, these neurons were shown to send signals to the zona incerta (ZI), a lesser-known brain area that has recently emerged as a key player in regulating arousal and sleep depth.
Watching the Brain Fall Asleep
In experiments using optogenetics—a technology that uses light to control genetically modified cells—researchers activated the REVglut2 neurons in live mice. The results were stunning: rather than instantly falling asleep, the mice exhibited drowsy behaviors reminiscent of the transition to rest in humans. They nested, groomed, and then slipped into a prolonged and deeply restorative sleep.
This pre-sleep behavior is significant. It suggests that these neurons not only facilitate sleep but may also control the brain’s preparatory stages for rest—a kind of neurobiological lullaby guiding the animal gently into unconsciousness.
To test whether these neurons were essential for rebound sleep—the phenomenon of catching up on rest after deprivation—the team chemically inhibited them during sleep-deprived states. Without the activity of the REVglut2 neurons, the mice failed to properly recover from their sleep debt. The restorative deep sleep that usually follows deprivation was blunted, and signs of fatigue lingered longer.
Further investigation revealed a critical link in the chain: when a key signaling protein called CaMKII—known to regulate neural plasticity and sleep drive—was blocked, the communication between the RE neurons and the zona incerta weakened, leading to significantly reduced recovery sleep.
Why It Matters: From Night Shifts to Neurodegeneration
Sleep is more than rest—it is repair. During deep NREM sleep, the body flushes metabolic waste from the brain, consolidates memories, and resets hormonal balances. Chronic sleep deprivation is now linked not only to poor concentration and mood disorders, but also to serious conditions such as Alzheimer’s disease, obesity, and cardiovascular problems.
Understanding the exact brain circuits that track sleep debt and promote recovery could pave the way for targeted therapies in a variety of populations. Shift workers, for instance—doctors, pilots, factory employees—often live in a state of semi-permanent jet lag, with circadian rhythms constantly disrupted. Similarly, people with insomnia or neurodegenerative diseases often experience fragmented or incomplete sleep.
“If we can figure out how to activate or protect this specific neuron population in humans,” said the lead neuroscientist of the study, “we might be able to restore healthy sleep patterns in people whose biological rhythms have gone off track.”
This could mean more than just improved rest. It might improve memory retention, reduce anxiety, and even delay the progression of diseases linked to poor sleep.
The Ticking of the Brain’s Sleep Clock
The idea that the brain keeps a detailed record of how long we’ve been awake—like a bank ledger of fatigue—has long fascinated scientists. But until now, the cellular mechanisms behind this sleep tracking remained elusive. This study offers the strongest evidence yet that certain neurons not only sense the length of wakefulness but also initiate the processes that lead to recovery.
It raises fascinating questions: Could we someday stimulate these neurons in people with sleep disorders to help them achieve deeper rest? Could pharmaceutical targets be developed that support REVglut2 activation without sedative side effects? Could sleep “recharge therapies” help those in high-stakes jobs that demand long hours?
Perhaps most intriguingly, the discovery opens a door to exploring whether similar neurons exist in humans—and if so, whether they can be gently nudged to provide better sleep without the grogginess associated with common sleep medications.
From Mice to Mankind: A New Chapter in Sleep Science
While this research was conducted in mice, the fundamental architecture of the mammalian brain is remarkably conserved. Many of the same circuits that control sleep in rodents exist in humans. The identification of REVglut2 neurons is therefore more than a mouse study—it is a glimpse into a universal mechanism of sleep regulation.
The work also represents the power of modern neuroscience. Techniques like optogenetics and viral tracing are allowing scientists to peer into living, thinking brains with unprecedented precision. They are uncovering not just where in the brain things happen, but how and why—a revolution in understanding the neural code behind human behavior.
The Poetry of Sleep, Revealed
Sleep is often dismissed as a passive state, something that happens when the brain shuts down. But in truth, sleep is a deeply active, biologically orchestrated performance. Neurons flicker with purpose. Chemicals rise and fall in waves. Circuits like those in the RE region work together to measure, anticipate, and finally deliver rest.
This new study doesn’t just find a new part of the map—it lights up a path we didn’t know existed.
As we understand more about these sleep-regulating neurons, we move closer to a future where rest is not a privilege or a struggle, but a guaranteed function of a well-tuned brain. A future where night-shift nurses and patients with Alzheimer’s alike might find a better night’s sleep—not through sedation, but through science.
And maybe, just maybe, we’ll finally answer one of the most enduring mysteries of all: not just why we sleep—but how we wake restored.
References: Sang Soo Lee et al, Sleep need-dependent plasticity of a thalamic circuit promotes homeostatic recovery sleep, Science (2025). DOI: 10.1126/science.adm8203. www.science.org/doi/10.1126/science.adm8203
Nicole M. Gilette et al, Where the brain pays sleep debt, Science (2025). DOI: 10.1126/science.ady6476. www.science.org/doi/10.1126/science.ady6476