At the heart of every cell in our body lies a remarkable story written in DNA. But this story is not endless—it comes with protective caps known as telomeres, fragile structures found at the tips of chromosomes. Telomeres act like the plastic ends of shoelaces, preventing our genetic material from unraveling. Each time a cell divides, telomeres become a little shorter, slowly ticking down the clock of cellular life.
For most of us, this process is gradual and natural, part of aging itself. But in some people, mutations disrupt the delicate balance of telomere maintenance, pushing cells into premature decline. When that happens, tissues stop regenerating, diseases emerge, and life becomes harder—sometimes as simple and essential as breathing.
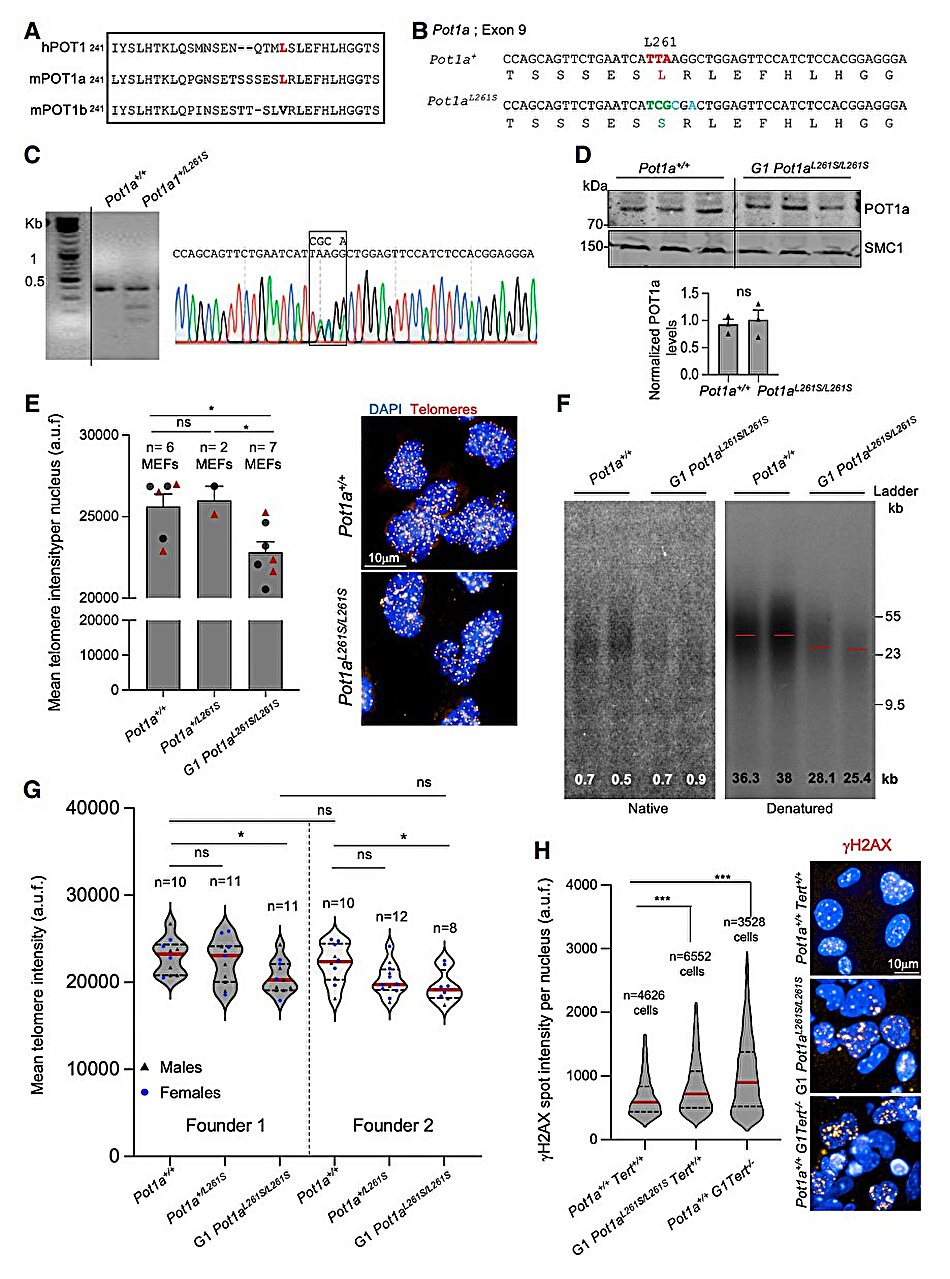
A new study by the Telomeres and Telomerase Group at Spain’s National Cancer Research Center (CNIO), led by Maria Blasco, uncovers how a mutation in the POT1 gene prevents lung tissue from renewing itself, ultimately leading to pulmonary fibrosis, a disease that leaves lungs scarred, stiff, and unable to function properly. Published in Genes and Development, this research does more than shed light on one mutation—it reshapes our understanding of how telomere dysfunction drives both degenerative diseases and cancer, and how therapies of the future may need to be as personalized as our DNA itself.
When Breathing Becomes a Struggle
Idiopathic pulmonary fibrosis (IPF) is a devastating diagnosis. Patients slowly lose the ability to draw breath as healthy lung tissue turns into stiff scar tissue. The disease is fatal, and despite years of research, there is still no cure.
Scientists have long suspected a strong link between pulmonary fibrosis and faulty telomeres. Shortened or dysfunctional telomeres prevent lung tissue from renewing itself. Over time, the body cannot replace damaged cells, and the lungs gradually lose their elasticity. Blasco’s group has been central to uncovering this connection, showing in earlier studies that reactivating telomerase—the enzyme that maintains telomeres—could reverse fibrosis in animals.
But the latest findings complicate this hopeful picture. The CNIO team discovered that when the POT1 gene, one of the so-called “shelterin” genes, mutates, telomeres become irreparably damaged. Even when telomerase is present, it cannot carry out its repairs. The result: fibrosis sets in, breathing grows harder, and the body’s most essential function is compromised.
POT1: A Guardian That Fails
To understand the impact of the POT1 mutation, one must appreciate the intricate machinery of telomere protection. Telomeres are not just stretches of DNA; they are safeguarded by a protein complex known as shelterin. POT1 is one of these crucial guardians.
Blasco’s team has shown that when POT1 mutates, it blocks telomerase from attaching to and repairing telomeres. “We have shown that this mutation stops telomerase from working in the telomere,” Blasco explains. Without telomerase’s help, telomeres shorten uncontrollably, cells stop dividing, and lung tissue fails to regenerate.
What makes this discovery striking is that, until now, POT1 mutations had been primarily associated with cancer. Cancer thrives when cells divide without limit, often because telomeres are somehow maintained despite normal shortening. But this same mutation can cause the opposite problem—premature tissue aging and degeneration, as in pulmonary fibrosis. In other words, the same gene can fuel both unchecked growth and irreversible decline.
Telomeres at the Crossroads of Cancer and Aging
The new findings underscore a paradox: the very structures that keep our DNA safe are also deeply implicated in disease. Short, dysfunctional telomeres accelerate aging by halting cell division, while abnormally long or unchecked telomeres allow cancer cells to divide endlessly.
The POT1 mutation, in particular, sits at this crossroads. Depending on its effects, it can tilt the body toward cancer or toward degenerative disease. “The fact that the same telomere protein, POT1, can lead to cancer or aging demonstrates the essential role of telomeres in these diseases,” says Blasco.
This dual role highlights why understanding telomere biology is not just academic curiosity—it is central to some of the most pressing health challenges of our time. Cancer and age-related diseases remain two of the leading causes of death worldwide. The possibility that a single pathway connects them offers both profound insight and new therapeutic opportunities.
The Promise and Limits of Telomerase Therapy
For years, telomerase activation has been hailed as a potential therapy for diseases linked to short telomeres, including pulmonary fibrosis. The CNIO spin-off company, Telomere Therapeutics, has been developing treatments that attempt to restore telomere length by stimulating telomerase in diseased tissues.
Yet the new findings from Blasco’s group suggest that this strategy will not work for everyone. In the case of POT1 mutations, even if telomerase is reactivated, it cannot do its job. The protective protein shield of the telomere is broken, and the repair machinery is effectively locked out.
This means therapies will need to be tailored to the genetic cause of disease—a personalized approach that considers not only whether telomeres are short but why they are short. For patients with telomerase mutations, telomerase activation may offer hope. For those with POT1 mutations, however, other strategies will be needed.
A Future of Personalized Medicine
The discovery of POT1’s role in pulmonary fibrosis is not just about one gene—it is a glimpse into the future of medicine. Diseases like fibrosis and cancer are not one-size-fits-all; they are shaped by the unique genetic makeup of each individual.
Blasco’s research highlights the importance of identifying the specific mutations driving disease in each patient. Only then can treatments be designed that target the root cause rather than the symptom. This approach, known as personalized or precision medicine, is becoming the new frontier of biomedical science.
For patients with pulmonary fibrosis, the stakes could not be higher. Every breakthrough brings us closer to therapies that can preserve lung function, extend lives, and restore the basic human act of breathing.
The Human Dimension of Scientific Discovery
Behind the technical details lies a deeply human story. To study POT1 is to confront the fragility of life itself—the way tiny molecular changes can determine whether lungs expand with ease or struggle for air, whether cells regenerate or fall into decline.
Research like this is not only about extending life but about preserving its quality. Breathing is the most fundamental rhythm of our existence, a reminder of both our vulnerability and our resilience. For those facing pulmonary fibrosis, each breath is hard-won. Understanding how a single gene like POT1 shapes this struggle is the first step toward easing it.
Conclusion: A Gene with Two Faces
The CNIO team’s discovery of the role of POT1 mutations in pulmonary fibrosis marks a milestone in telomere biology. It reveals how the failure of a single protein can prevent telomeres from repairing, leading to scarring lungs, lost breath, and shortened lives. At the same time, it emphasizes the paradoxical role of telomeres as guardians of both longevity and vulnerability, capable of driving cancer in one context and degenerative disease in another.
The future of treatment lies in personalization—recognizing that no two patients are alike, and that therapies must be tailored to the precise genetic cause. With each discovery, science moves closer to making this vision a reality.
In the end, physics explains the laws of the universe, but biology explains the fragility of life within it. And in the tiny ends of our chromosomes—the telomeres—we find both the secrets of aging and the key to some of humanity’s most devastating diseases. The story of POT1 is not just about genes; it is about our shared struggle for breath, and the hope that science can restore it.
More information: Raúl Sánchez-Vázquez et al, Mice carrying the homologous human shelterin POT1-L259S mutation linked to pulmonary fibrosis show a telomerase deficiency-like phenotype with telomere shortening with increasing mouse generations, Genes & Development (2025). DOI: 10.1101/gad.352855.125