For the first time in history, scientists have managed to watch the living body’s lifeblood move in real time—through entire organs, down to the tiniest vessels. This remarkable feat, achieved by a team from France’s Physics for Medicine Institute (Inserm/ESPCI Paris-PSL/CNRS), represents a major leap forward in biomedical imaging. Using an innovative ultrasound technique, researchers have mapped blood flow in animal organs—heart, kidney, and liver—in four dimensions: three-dimensional space and time.
Published in Nature Communications, this achievement opens a window into one of the most intricate systems of the body—the microcirculation that nourishes every cell and keeps us alive. The technique, if successfully adapted for human use, could transform how doctors understand and diagnose cardiovascular and metabolic diseases, offering new hope for millions worldwide.
The Hidden Highways of Life
Blood microcirculation is often called the body’s “hidden highway.” It’s a vast and delicate network of tiny blood vessels—arterioles, capillaries, and venules—that connect large arteries and veins to every living tissue. Through these slender channels, oxygen and nutrients reach cells, and waste products are carried away for removal.
When everything flows smoothly, organs thrive. When something goes wrong—when a vessel narrows, leaks, or clogs—the results can be devastating. Heart failure, kidney failure, diabetes, strokes, and chronic inflammation all have their roots in disrupted microcirculation.
Yet despite its critical role, this fine-scale network has remained largely invisible. Traditional imaging tools like MRI, CT scans, and standard ultrasound can show large blood vessels but not the smallest ones, where disease often begins. The problem has long been one of scale—how to see both the forest and the trees.
A Revolutionary Way to See Blood Flow
That challenge is what the Inserm team set out to solve. Their breakthrough came in the form of a new kind of ultrasound probe—an elegant instrument that can capture images of the entire organ, while simultaneously tracking blood movement in microvessels smaller than a strand of hair.
Developed under the supervision of Inserm researcher Clément Papadacci as part of Nabil Haidour’s doctoral work, the technology pushes ultrasound far beyond its traditional limits. Instead of flat, gray cross-sections, it produces vivid, 4D renderings—three spatial dimensions plus time—of how blood actually flows through complex vascular networks.
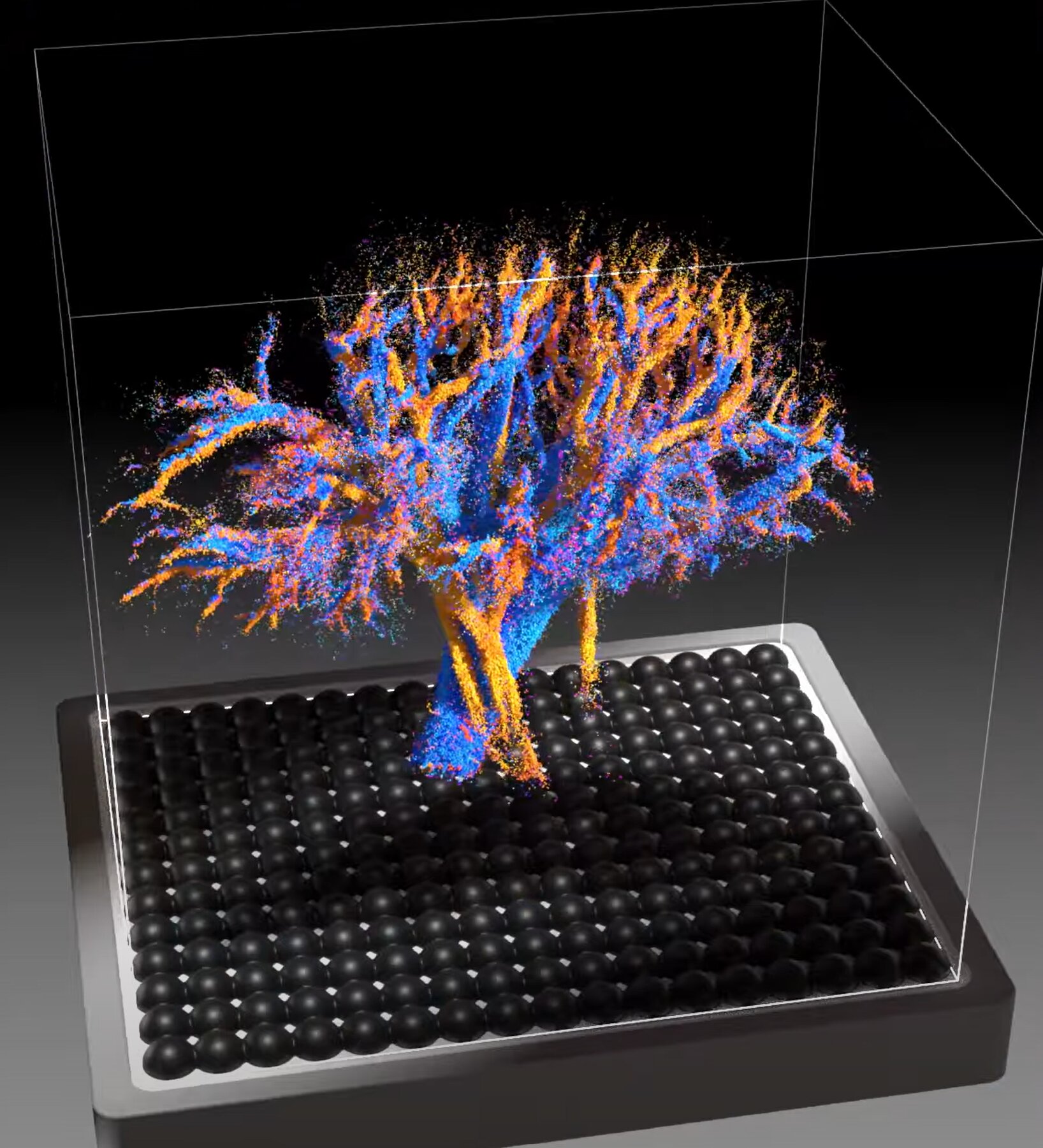
In their study, the researchers tested the technique on animal organs similar in size to human ones. The images they produced were astonishing. In the heart, they could visualize blood coursing through intricate layers of muscle tissue. In the kidneys, they observed the fine branching structures that filter blood and regulate fluid balance. And in the liver, they could not only map its architecture but also distinguish between its three distinct vascular systems—arterial, venous, and portal—each with its unique hemodynamic “signature.”
According to Papadacci, “The originality of these results lies in the fact that these images allow us to visualize the vessels of an entire organ at very small scales—less than 100 micrometers. This 4D image resolution is unprecedented, as is the ability to observe an entire large organ and its flow dynamics.”
Seeing the Body in Motion
The “fourth dimension”—time—is what truly sets this technique apart. Traditional imaging captures static snapshots of anatomy, but the body is not static. Blood pulses, pressure changes, and flow patterns evolve every millisecond. By adding time to the picture, the researchers have given science a moving portrait of the body’s inner life.
This dynamic view offers more than just beauty. It reveals how blood interacts with tissues, how flow changes during disease, and how treatments alter circulation. For clinicians, this could be a game-changer. Instead of inferring problems indirectly, doctors could watch them unfold directly, in real time.
From Lab to Clinic
The next step for the research team is to bring this technology from animal models to human patients. Early preparations for clinical trials are already underway, supported by the Technological Research Accelerator for Biomedical Ultrasound, a collaboration between Inserm and the Physics for Medicine Institute.
One of the most promising aspects of this innovation is its accessibility. The probe can connect to small, portable ultrasound systems—devices that are already staples in hospitals and clinics worldwide. This means the technology could be integrated into daily medical practice without the need for massive, expensive new infrastructure.
“Used in clinical settings,” explains Papadacci, “this new technology could become a major tool for better understanding vascular dynamics as a whole, from the largest vessels to the pre-capillary arterioles. It could also help advance the diagnosis of microcirculation disorders and the monitoring of treatments for small vessel diseases, which are complex to diagnose and are diagnosed by ruling out other pathologies.”
In other words, this tool could shift medicine from reactive to proactive—catching diseases in their earliest stages, when interventions are most effective.
Why It Matters for Human Health
The potential implications of this innovation are vast. Many common and deadly diseases—from heart disease and diabetes to dementia—are linked to tiny blood vessel dysfunction. Yet current imaging tools often miss these changes until it’s too late.
Being able to see microcirculation in real time could allow doctors to detect early warning signs of disease, track how well a treatment is working, or understand why some therapies fail. For example, oncologists could study how blood flow within tumors affects drug delivery. Cardiologists could observe how small vessel disease develops before it leads to a heart attack. Nephrologists could monitor kidney perfusion during chronic conditions or after transplantation.
Beyond medicine, the insights gained from this technology could deepen scientific understanding of human physiology itself. By visualizing how the body regulates its own blood flow, researchers might uncover new biological principles that govern health and aging.
A Fusion of Physics and Medicine
At its core, this breakthrough is as much a triumph of physics as of medicine. It exemplifies how collaboration between disciplines can yield discoveries that neither could achieve alone.
The Physics for Medicine Institute was created precisely for this purpose—to bring together physicists, engineers, and medical researchers who view the human body as both a biological system and a complex physical structure. Their shared mission is to use the laws of physics to explore life, improve health, and design the next generation of medical technologies.
The 4D microcirculation imaging project embodies this mission perfectly. It translates abstract concepts like wave propagation and acoustic scattering into practical tools that can save lives. It shows that when science crosses boundaries, the results can be transformative.
The Future of Medical Imaging
If successful in humans, this technology could usher in a new era of medical imaging—one where doctors no longer guess how blood moves inside organs but see it directly, in motion and detail. It could become a universal diagnostic lens through which vascular health is evaluated with precision and clarity.
The dream, according to the Inserm team, is to eventually make the probe a standard part of clinical ultrasound systems, available to any hospital or clinic. Because it is non-invasive, safe, and portable, it could be used not only in advanced research centers but also in local hospitals and emergency rooms.
Imagine a physician being able to scan a patient’s heart or kidney and instantly visualize not just its structure but how blood is flowing through it, second by second. Such capability would revolutionize preventive care, accelerate diagnosis, and personalize treatment.
More information: Multi-lens ultrasound arrays enable large scale three-dimensional micro-vascularization characterization over whole organs, Nature Communications (2025). DOI: 10.1038/s41467-025-64911-z