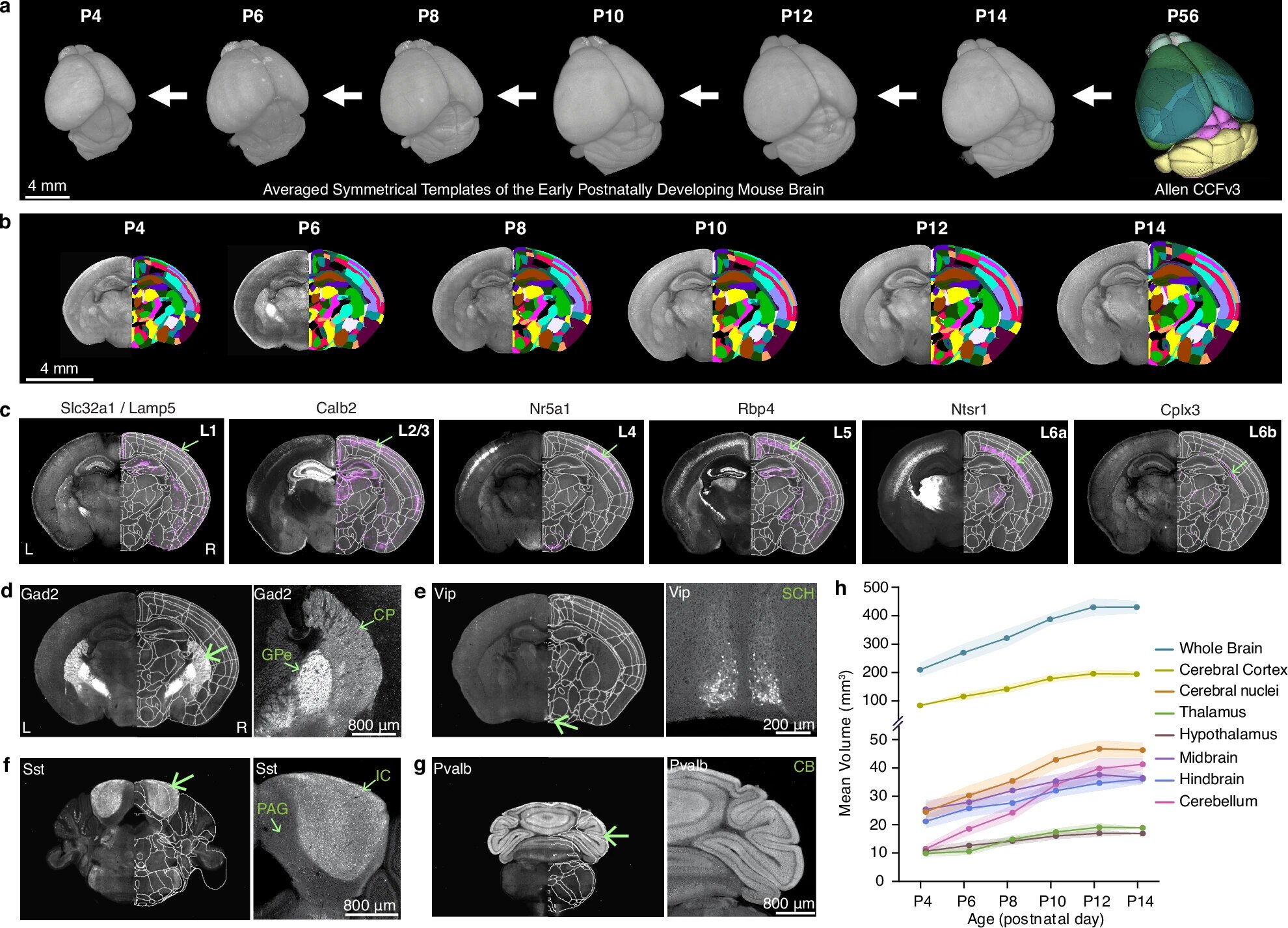
The brain does not grow like a staircase—step by step, predictably. Instead, it unfolds like a living symphony, where countless parts develop in harmony and respond to both internal genetic cues and the outside world. A groundbreaking new study by researchers at Penn State College of Medicine and the Allen Institute for Brain Science has now captured this intricate dance in unprecedented detail.
By building the first high-resolution, three-dimensional growth chart of the developing mouse brain, the team has offered scientists a powerful new tool to understand how the brain matures—and what happens when that process goes wrong. The findings, published in Nature Communications, bring us closer to understanding the origins of neurodevelopmental disorders such as autism, which often emerge during early brain development.
A Dynamic Picture of Brain Development
For decades, scientists have tried to map how the brain changes as it grows, but previous models were often low in resolution—like blurry photographs where only large structures could be seen. The new 3D atlases, however, reveal growth at the level of individual cells, creating what the researchers describe as “a high-definition photo” of the developing brain.
“Brain growth doesn’t proceed in a simple linear way,” explained Yongsoo Kim, professor of neuroscience and experimental therapeutics at Penn State College of Medicine and senior author of the study. “It’s a complex, choreographed process that responds to genes and the environment—things like light, sound, and touch.”
To capture this dynamic process, Kim’s team imaged the entire mouse brain every other day from postnatal day four to day fourteen—a period equivalent to late pregnancy through early childhood in humans. Using a cutting-edge imaging method called serial two-photon tomography, they scanned the brain at microscopic resolution, generating vivid three-dimensional maps that show not just where growth happens, but how it unfolds across time.
Why the Mouse Brain Matters
While the mouse brain is much smaller than the human brain, it is surprisingly similar in its basic structure and chemistry. Many of the same neural circuits that underlie movement, emotion, learning, and perception are conserved across mammals. That makes the mouse an ideal model for studying the principles of brain development that also apply to humans.
In the first two weeks of life, the mouse brain undergoes rapid transformation. Neurons form new connections, networks strengthen, and sensory systems awaken. It’s a time of extraordinary plasticity—the ability of the brain to adapt and rewire in response to experience.
“This is also when many neurodevelopmental disorders begin to take shape,” Kim explained. “If something goes wrong in this early period—whether because of genetics, environment, or both—it can ripple through the entire process of brain maturation.”
Where the Brain Grows Fastest
The researchers discovered that not all regions of the brain grow at the same rate. Among all the areas studied, the cerebellum—the structure at the back of the head responsible for coordinating movement and balance—showed the greatest increase in volume during the early postnatal period.
This finding aligns with what scientists know about human development: the cerebellum not only fine-tunes motor skills but also contributes to cognitive functions such as attention, language, and emotional regulation. Its rapid growth makes it both powerful and vulnerable—a hotspot for developmental change and, potentially, for disruption.
Tracing the Brain’s “Brake System”
Beyond mapping volume changes, the study focused on two specific types of brain cells that play critical roles in shaping neural circuits: GABAergic neurons and microglia.
GABAergic neurons are the brain’s inhibitory cells—the ones that act like brakes, preventing excessive activity and maintaining balance within neural networks. They are essential for stable communication between different parts of the brain.
The team observed that these neurons show strikingly different patterns in different brain regions. In the cortex—the brain’s outer layer that processes sensory input and complex thought—the density of GABAergic neurons decreased sharply, leveling off around postnatal day twelve. Meanwhile, their density increased in the striatum, a deeper structure involved in movement and motivation.
This suggests that GABAergic neurons are not fixed in place from birth. Instead, they dynamically reorganize as the brain matures, responding to the needs of each region. Kim noted that many neurodevelopmental disorders, including autism and epilepsy, are linked to problems in this “brake system.” Understanding how and when these neurons develop could reveal new insights into how those disorders arise.
The Brain’s “Gardeners”: Microglia and the Pruning of Connections
If GABAergic neurons are the brain’s brakes, microglia are its gardeners. These immune cells patrol the brain, clearing away debris and pruning unnecessary connections between neurons. This pruning process is vital for shaping efficient, well-functioning neural networks.
The researchers found that microglia populations shift dramatically during the first two weeks of life. Until postnatal day eight, they are concentrated in the white matter—the brain’s internal wiring that connects distant regions. But around day ten, their numbers in the white matter drop sharply as they migrate into the gray matter, which contains the neurons responsible for processing information.
Even more intriguingly, microglia became denser in regions that process sensory information just as the mice began to open their eyes and ears. “We don’t yet know exactly what drives this shift,” Kim said, “but it suggests that microglia may respond to sensory experience—sight, sound, and touch—helping to fine-tune brain wiring as the animal starts interacting with the world.”
A Window Into the Brain’s Earliest Moments
The study’s findings paint a vivid picture of early brain development as a fluid, responsive process. Cells move, grow, and adapt as the brain expands and begins to sense the outside world. The timing of these changes appears crucial—any disturbance during this window could have cascading effects.
“This early postnatal period is like laying the foundation of a house,” Kim said. “If something goes wrong with the foundation, even small problems can affect the whole structure later on.”
By pinpointing when and where key changes occur, the team’s high-resolution atlas could help scientists understand what happens in disorders where development goes awry. For instance, if inhibitory neurons fail to populate the right regions or if microglia prune too aggressively—or not enough—it could alter the balance of neural activity and contribute to conditions like autism, ADHD, or schizophrenia.
A Tool for Collaboration and Discovery
To maximize the impact of their work, the researchers made their 3D brain atlases and growth charts publicly available through an interactive online platform. This allows scientists worldwide to explore the data, compare it with their own findings, and build on the framework to study other aspects of brain development.
“The real significance of this work,” Kim explained, “is that it provides a spatial framework for integrating different types of data—molecular, cellular, and anatomical. It’s a foundation for a more complete picture of how the brain develops.”
By sharing their results openly, the team hopes to accelerate discoveries not only in basic neuroscience but also in medicine. The more precisely scientists can map the normal trajectory of brain development, the better they can recognize when and how it goes off course.
The Promise of a Sharper View
The high-resolution atlas developed by Kim’s team represents more than a technical achievement—it’s a leap forward in how we see the brain itself. Each cell, each layer, each connection contributes to a living, evolving system that adapts in real time to the world it encounters.
For centuries, scientists could only glimpse this complexity indirectly. Now, through advances in imaging and computation, we can watch it unfold with clarity and depth never before possible.
In doing so, we begin to grasp the immense beauty and precision of brain development—the balance of growth and restraint, pruning and connection, structure and function. And in that understanding lies hope: the hope that by seeing the brain’s earliest steps more clearly, we can better protect it when things go wrong.
More information: Josephine K. Liwang et al, epDevAtlas: mapping GABAergic cells and microglia in the early postnatal mouse brain, Nature Communications (2025). DOI: 10.1038/s41467-025-64549-x