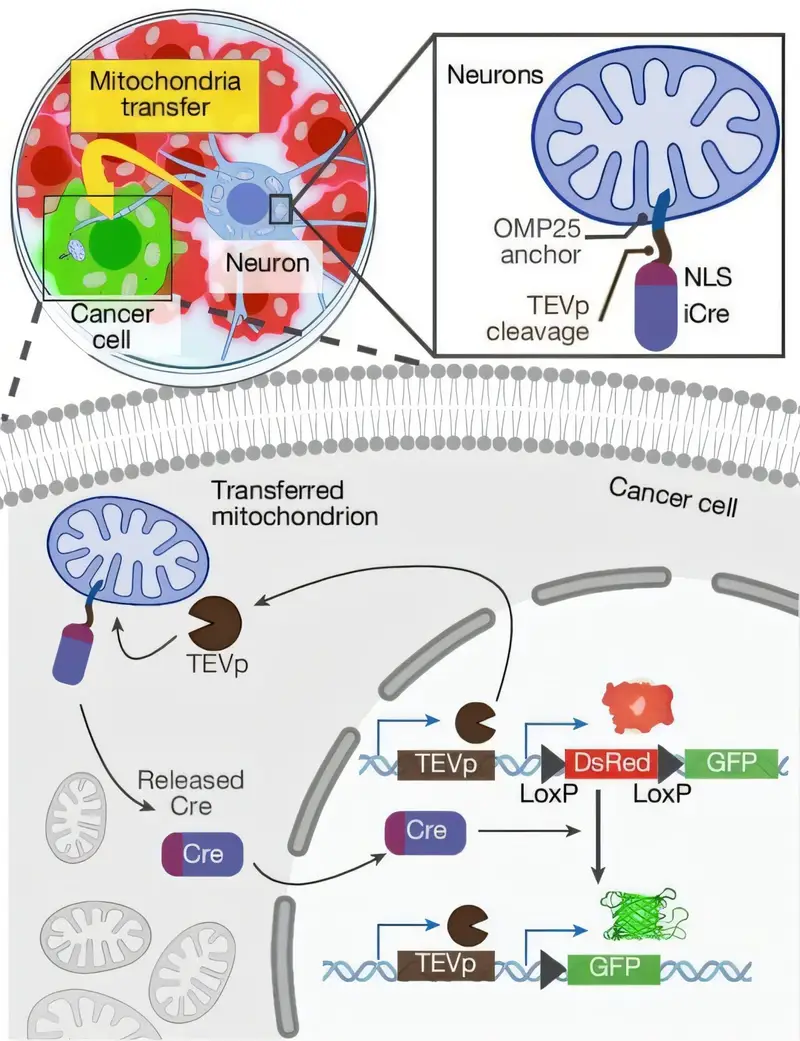
Cancer has long been a master of disguise and deception—tricking immune cells, hijacking blood vessels, and reshaping its environment to survive. But now, scientists have uncovered one of its most astonishing collaborators yet: the nervous system itself.
In a landmark study published in Nature, researchers from the University of South Alabama have discovered that neurons can transfer their own mitochondria—tiny energy-generating organelles—to cancer cells. This donation isn’t just symbolic. It powers up the tumor cells, boosting their metabolism, making them stronger, and helping them spread more aggressively throughout the body.
It’s a chilling partnership, hidden deep within the tissues of our bodies. And it’s changing the way we understand cancer—not just as a disease of rogue cells, but as a hijacker of our biology’s most fundamental support systems.
An Unlikely Alliance: Neurons and Tumors
For years, oncologists have noticed a strange trend: tumors that grow nestled in dense webs of nerves often behave more aggressively. They grow faster, resist treatment, and are more likely to metastasize. This observation sparked an unsettling question—what if nerves are helping tumors?
Past studies gave clues. When scientists severed nerve connections to tumors in rodents or humans, cancer growth often slowed. But how, exactly, were nerves influencing cancer cells? Until now, that remained a mystery.
The new study, titled “Nerve-to-cancer transfer of mitochondria during cancer metastasis,” offers a powerful answer.
Mitochondria: Life’s Tiny Engines
To understand what the researchers found, we need to visit the microscopic world inside every cell. Mitochondria are often called the “powerhouses” of the cell. These bean-shaped structures generate energy through a process called oxidative phosphorylation, fueling the cell’s activity and survival.
Under normal circumstances, healthy cells can donate mitochondria to struggling neighbors—a kind of cellular charity. Astrocytes in the brain, for example, can pass mitochondria to damaged neurons after injury. This transfer helps revive energy-starved cells and restore function.
But what happens when this gift is exploited?
Two Sides of the Experiment
To investigate this question, the researchers used mouse models of breast cancer. They designed two sets of experiments to explore how nerves interact with tumors.
Disabling the Nerves
In the first, they chemically shut down nerve activity around breast tumors using botulinum neurotoxin A (BoNT/A)—a method known as chemical denervation. This silenced the nerves’ input, cutting off any potential assistance to nearby cancer cells.
The results were dramatic. Tumors with disabled nerve connections had fewer mitochondria, lower expression of key metabolic genes, and a significantly reduced rate of invasion—only 12% of the denervated tumors formed invasive lesions, compared to 55% in the untreated group.
In short, without nerves, the tumors starved.
Lighting Up the Transfer
In the second experiment, the team went even deeper. They engineered neurons to express fluorescently labeled mitochondria using a technique they called MitoTRACER. This advanced tool allowed the scientists to watch mitochondria move from neuron to cancer cell in real time.
And that’s exactly what they saw.
Through long, threadlike structures called tunneling nanotubes, neurons physically transferred mitochondria into cancer cells. Once inside, the mitochondria didn’t just sit there—they fired up.
The recipient tumor cells, once deprived of their own mitochondrial function, suddenly regained the ability to perform oxidative phosphorylation, the process critical for efficient energy production. Even more strikingly, they began to grow without external support like uridine, a vital nutrient. They had been functionally rescued.
The Gift That Empowers
The implications of this mitochondrial donation were profound. The cancer cells that received neuronal mitochondria showed:
- Higher ATP production (more energy)
- Improved resistance to oxidative stress
- Greater survival under mechanical pressure
- Enhanced mobility and invasion potential
When researchers traced these energized cells in animal models, they found something even more disturbing—they were overrepresented in distant metastases, particularly in the brain and liver. Neuronal mitochondria had not only powered up the tumor cells but also helped them travel and colonize new tissues.
Who’s Pulling the Strings?
While neurons may appear to be the benefactors in this biological transaction, the researchers believe it’s the cancer cells that initiate the relationship.
“Rather than neurons sensing distress and offering help,” the team wrote, “our findings suggest that cancer cells actively recruit and instruct neurons to donate mitochondria.”
This shifts the narrative from accidental rescue to intentional manipulation. Cancer, it seems, knows how to ask—and take—what it needs.
The New Battlefield: Nerves as a Target
This discovery opens the door to a potentially revolutionary approach to cancer therapy: targeting the nervous system.
If tumors rely on neural connections not just for growth signals but for metabolic machinery itself, then disrupting these connections could strip cancer of one of its key survival strategies.
Chemical denervation, nerve-blocking drugs, or interventions that prevent mitochondrial transfer might become part of the next generation of anti-cancer treatments. Not by killing the cancer directly, but by cutting off its supply lines.
What Comes Next
As with any breakthrough, this study raises as many questions as it answers. How exactly do cancer cells signal neurons to begin mitochondrial transfer? Can this behavior be observed across all types of tumors, or is it specific to certain cancers like breast or brain? Could mitochondrial transfer explain why some cancers are resistant to therapy, or why they return after treatment?
The researchers caution that while the findings are compelling, more research is needed to map the full implications. Yet even in its early stages, this work has already redefined a fundamental truth about cancer: it doesn’t act alone.
It recruits. It communicates. It manipulates the very systems meant to support life, turning them into accomplices in its deadly quest.
A New Kind of War
In the intricate and often invisible war waged inside our bodies, cancer has always been a formidable enemy. But now, we understand that it’s not just fighting with its own weapons—it’s borrowing ours.
The nervous system, the very network that lets us think, feel, and survive, can also be twisted into a tool for destruction. The discovery that neurons can hand over mitochondria—the engines of life—to cancer cells changes everything we thought we knew.
It’s a sobering realization. But it’s also a powerful call to action.
Because the more we uncover cancer’s secrets, the more vulnerable it becomes.
And somewhere in the tiny glow of a stolen mitochondrion, we may just find the next way to fight back.
Reference: Gregory Hoover et al, Nerve-to-cancer transfer of mitochondria during cancer metastasis, Nature (2025). DOI: 10.1038/s41586-025-09176-8