Imagine losing your head—and growing it back.
In the mysterious world of planarian flatworms, this isn’t science fiction. It’s biology. And recent research from the National Cancer Institute and its partners has revealed that this astonishing ability isn’t simply switched on from birth—it emerges gradually, unfolding during the earliest chapters of the worm’s life. What scientists have now uncovered in Schmidtea polychroa, a flatworm species known for whole-body regeneration, could reshape how we understand tissue renewal—and perhaps even human healing—in the future.
This new study dives deep into the flatworm’s biology, uncovering the moment when regeneration switches from limited patchwork repair to full-blown reconstitution of body structures. And it’s not just about having stem cells. As it turns out, the ability to reset the body’s axis—to remember which way is up and which is down—acts as a gatekeeper to the flatworm’s full regenerative potential.
Regeneration: Nature’s Masterstroke
Regeneration is one of biology’s most jaw-dropping tricks. From starfish that regrow lost arms to salamanders that restore entire limbs, life has evolved myriad ways to rebuild itself. But there’s a wide range in how, and how much, creatures can regenerate.
For humans, regeneration is mostly limited to healing cuts, broken bones, and some internal linings. Our livers can regrow to an extent. Children’s fingertips sometimes regrow if sliced off at just the right spot. But regrowing a limb? That remains in the realm of imagination—or, at least for now, in the realm of worms.
In stark contrast, aquatic creatures like hydrozoans, planarians, and sponges can regrow entire bodies from fragments that seem too small to contain a full blueprint. In these animals, regeneration is less of a miracle and more of a routine biological process.
But even in these elite regenerators, the story is more nuanced. Age, environment, and developmental timing all play a role in determining how much can be regrown—and how well.
The Flatworm That Rewrites Itself
Among the superstars of regeneration, planarian flatworms shine brightest. Slice one in half, and it will grow a new head or tail depending on what’s missing. Chop it into pieces, and each one can become a new worm. Their secret? A unique population of adult stem cells known as neoblasts.
Neoblasts are distributed throughout the planarian body. These pluripotent cells can become virtually any cell type, springing into action after injury, during reproduction, or simply to replace aging tissues. But neoblasts, impressive as they are, don’t work alone. They rely on signals—biochemical instructions that tell them what to become and where to do it. Without these positional cues, even the most potent stem cells are aimless.
That’s where axis reset comes into play.
The Moment Regeneration Awakens
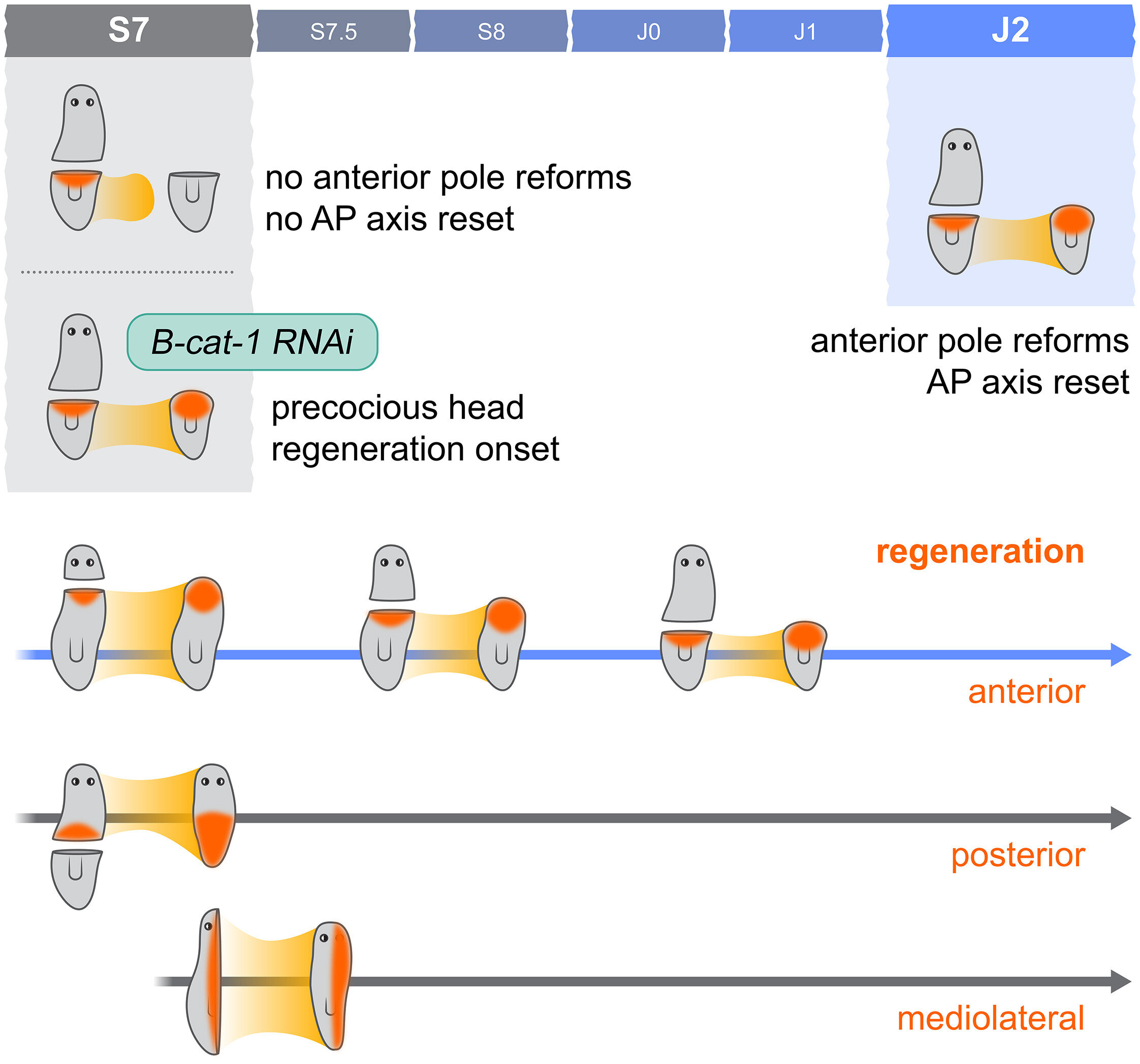
In the new study, researchers set out to determine when in its development Schmidtea polychroa becomes capable of regenerating an entire body. Is this ability present from the embryo’s first moments? Or does it unfold as the worm matures?
To find out, scientists staged embryos and juveniles at defined points in their development and then performed carefully planned amputations. By bisecting worms along the anterior-posterior (head-to-tail) axis, they could observe which parts regrew and when.
The results were striking: posterior tissues (tails) regrew earlier than heads. In the earliest stages, the worm could regenerate a tail from the head side, but not the other way around. Head regeneration lagged behind, only appearing later—after hatching, in fact.
The conclusion? Whole-body regeneration is a staged capability, not an automatic one. And it hinges on more than just having stem cells. Something else—a biological checkpoint—needs to be passed first.
The Axis Reset: More Than Just Direction
So what exactly is this axis reset?
Every organism has a body plan—a blueprint that determines where the head goes, where the tail belongs, and how organs are arranged. In development, this body axis is laid down early. But after injury, that axis can become scrambled, especially in small body fragments. Resetting it is like reloading the master plan.
In S. polychroa, the capacity to regenerate a head only appeared after the animal gained the ability to re-establish this polarity. Without it, no matter how many stem cells were present, head regeneration simply didn’t happen.
To test the importance of this axis reset, scientists performed a remarkable experiment: they silenced β-catenin-1, a protein critical in the Wnt signaling pathway, known to suppress head formation. When they knocked down β-catenin-1 in fragments that normally couldn’t regenerate heads, voila—head regeneration was restored.
This discovery strongly suggests that regeneration is not solely a question of resources (stem cells), but one of biological context—having the right signals at the right time to direct the rebuilding.
Stem Cells Alone Are Not Enough
To further probe the regenerative mystery, researchers applied irradiation, which destroys rapidly dividing cells such as neoblasts. As expected, regeneration failed in irradiated worms. But curiously, simply having unirradiated, stem cell–rich tissue wasn’t enough either. Unless the axis reset mechanism was intact, no amount of neoblasts could fix the problem.
In other words, regeneration is a team sport, requiring both the players (stem cells) and the coach (positional signals). And without the coach’s game plan, the players don’t know what to do.
Rethinking Regeneration Across Life Forms
The implications stretch far beyond worms. Across the animal kingdom, regenerative capacity varies dramatically—and often declines with age. In frogs like Xenopus, limb and tail regeneration is robust in tadpoles but fades in adults. Mice can regrow digit tips as juveniles, but not as adults. Even in tunicates, sponges, and annelids, aging blunts the regenerative edge.
These changes have been blamed on several factors: the depletion of stem cells, loss of cell flexibility (plasticity), epigenetic changes that lock genes into fixed patterns, and metabolic shifts that alter cellular behavior. But the S. polychroa study introduces a new angle—perhaps the loss of regeneration isn’t about losing stem cells, but about losing the signals that tell cells how to act.
Interestingly, some species buck the trend. Sponges, ctenophores, and certain crinoids show improved regenerative abilities in adulthood. And planarians like S. polychroa retain full-body regenerative capacity through life, as long as their signaling networks remain functional.
Healing in Humans: Lost Art or Sleeping Potential?
So where does this leave us? Humans are notoriously bad at regeneration. We patch injuries with scar tissue. We heal quickly—but not precisely. Our bodies prioritize fast sealing over perfect reconstruction. It’s a trade-off, evolutionarily speaking. In large, complex animals, stopping bleeding fast can be the difference between life and death.
But what if the capability to regenerate isn’t lost—just dormant?
The flatworm study suggests that regeneration isn’t about having special cells that other animals lack. It’s about reactivating pathways that determine body identity, axis orientation, and injury response. If we could learn how to reset the body’s axis—or mimic the signals that drive flatworm regeneration—could we unlock similar potential in mammals?
Beyond the Worm: A Regenerative Future
Already, scientists are exploring how to apply these principles to regenerative medicine. Research into Wnt signaling, epigenetic reprogramming, and stem cell engineering seeks to emulate the regeneration seen in planarians.
But this new study offers a crucial reminder: even the most regenerative cells won’t help unless they know what to do. Regeneration is conditional, contextual, and deeply tied to developmental logic.
The idea that head regeneration in flatworms is not an inherent trait but an acquired competence changes the narrative. It tells us that regenerative potential is not a yes-or-no trait—it’s a switch that can be flipped under the right conditions.
Perhaps the key to healing lies not in inventing something new, but in remembering something ancient—something that evolution has obscured, but not erased.
Reference: Clare L.T. Booth et al, Developmental onset of planarian whole-body regeneration depends on axis reset, Current Biology (2025). DOI: 10.1016/j.cub.2025.03.065