For more than a century, the scientific consensus was ironclad: neural stem cells (NSCs)—those remarkable, self-renewing cells capable of transforming into neurons and glial cells—existed solely within the sanctum of the central nervous system (CNS), confined to the brain and spinal cord. This dogma was so entrenched that few dared to question it. But now, in a surprising twist, a multinational team of scientists led by Hans Schöler from the Max Planck Institute for Molecular Biomedicine has shattered this long-standing belief. Their groundbreaking research, published in Nature Cell Biology, unveils a new, elusive type of neural stem cell existing outside the CNS—aptly named peripheral neural stem cells (pNSCs). And their discovery might just be the missing key in the future of neurological repair.
From Failure to Fortune: The Accidental Discovery
Ironically, the origin of this breakthrough traces back to a scientific controversy that once promised too much. In 2014, a sensational article titled “Stimulus-triggered fate conversion of somatic cells into pluripotency” claimed that simple acidic stress could reprogram adult cells into pluripotent stem cells. It was a revolutionary notion—no genetic modification, no viral vectors, just a splash of low pH and voilà! A stem cell. The world took notice. But as labs across the globe, including Schöler’s, scrambled to replicate the findings, results repeatedly failed. The paper was soon retracted, leaving behind a trail of skepticism and disappointment.
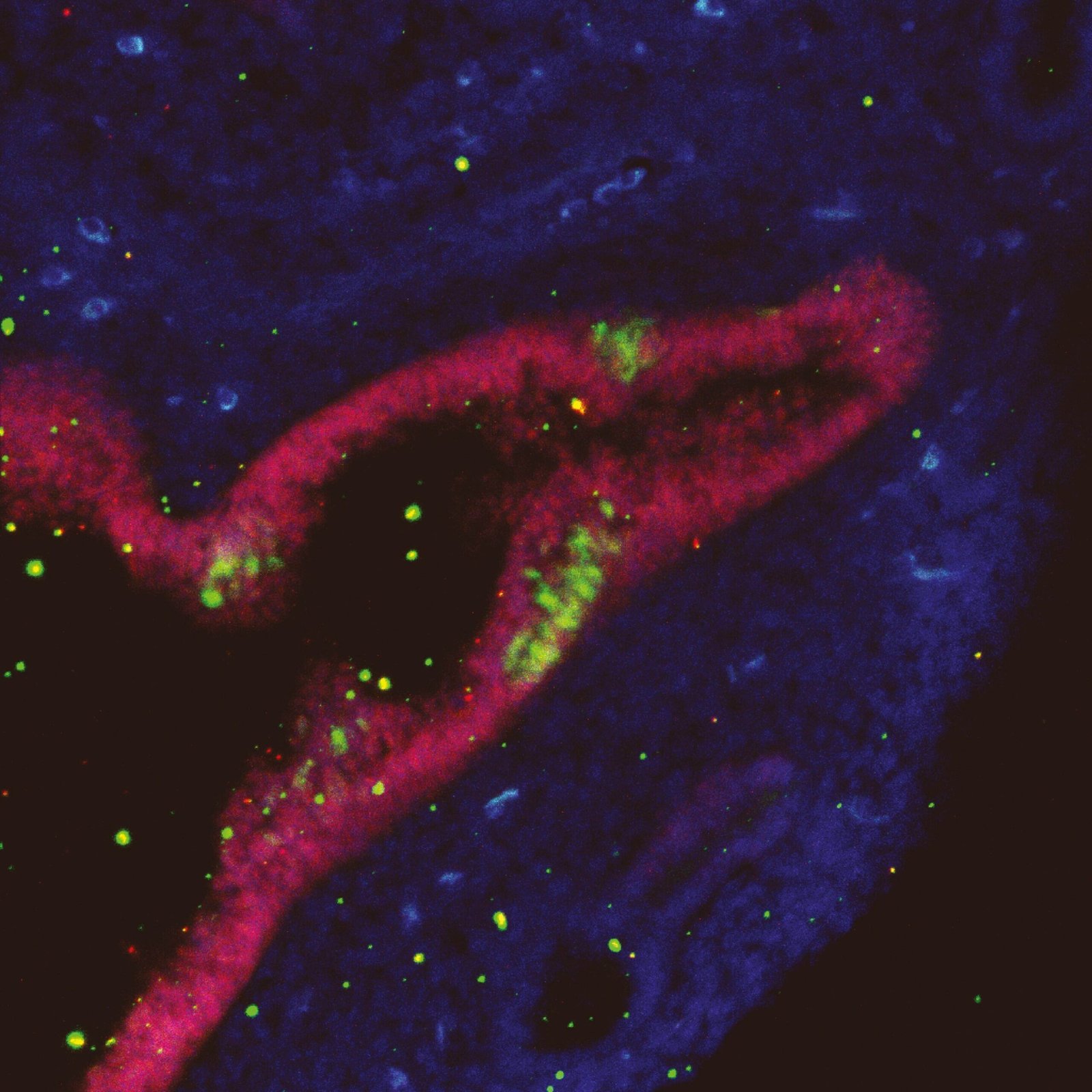
But Schöler’s team didn’t abandon their experiments. Lead researcher Dong Han, while probing mouse tissues under low pH conditions, began noticing a peculiar and rare group of cells emerging—not pluripotent cells, but something strikingly similar to neural stem cells. Intriguingly, these cells weren’t from the brain or spinal cord. They were appearing in tissues as far-flung as the lung and tail. And thus began the long and arduous journey that would eventually identify and confirm the existence of pNSCs.
Peripheral Neural Stem Cells: A New Class of Cellular Architect
Unlike the fleeting existence of many stem-like cells that often fail to meet the rigorous benchmarks of true stemness, pNSCs check nearly every box. Morphologically, they look like brain NSCs. Functionally, they behave like brain NSCs—self-renewing and capable of differentiating into neurons and glia. They express key NSC-specific markers, and their transcriptional and epigenetic profiles closely match those of CNS-derived NSCs.
The pNSCs’ behavior was scrutinized across an international collaboration spanning more than 10 labs from Europe, North America, and Asia. The findings were nothing short of revelatory: these cells not only share a molecular identity with traditional NSCs, but during embryonic and postnatal development, many are capable of migrating from the neural tube to peripheral tissues—where they retain their ability to transform into mature neurons and glial cells.
This suggests a new layer of cellular plasticity in the mammalian nervous system—one that’s been hiding in plain sight.
Why This Matters: A Paradigm Shift in Neuroscience
The discovery of pNSCs is not merely academic—it fundamentally changes how scientists understand the development and repair potential of the nervous system. For decades, the isolation of NSCs required the dissection of brain tissue, a process fraught with ethical, technical, and biological limitations. Brain-derived NSCs are difficult to harvest, fragile outside their native environment, and notoriously resistant to large-scale expansion.
pNSCs, in contrast, offer a practical alternative. Their presence in peripheral organs means they could potentially be accessed with far less invasive procedures. Even more tantalizing is the fact that they can be grown robustly in culture—an essential property for any cell type being considered for therapeutic application.
Regenerative Medicine on the Horizon
If pNSCs exist in humans—and early indications suggest this is likely—then their therapeutic potential could be immense. These cells could be harvested from a patient’s own peripheral tissue, expanded in the lab, and reintroduced to repair damaged neural circuits in conditions such as:
- Parkinson’s disease: Replacing the lost dopamine-producing neurons could alleviate symptoms and restore function.
- Spinal cord injury: Grafting pNSCs at the injury site might aid in rebuilding severed neural connections.
- Alzheimer’s disease: Although complex, neuroregeneration using patient-derived stem cells could be a future strategy.
And because pNSCs mirror the behavior of CNS NSCs so closely, their use in clinical settings would avoid many of the pitfalls associated with other stem cell types, such as embryonic stem cells or induced pluripotent stem cells (iPSCs), which may pose tumorigenic or immunological risks.
The Science Behind the Claim: Proving the Existence of pNSCs
To convince the scientific community of the legitimacy of pNSCs, the team employed a multi-pronged approach. Using in vivo lineage tracing, they demonstrated that these cells originate from the neural tube and migrate into peripheral tissues during development. Single-cell RNA sequencing revealed their transcriptional similarity to canonical NSCs, while epigenetic analysis confirmed the activation of key neural developmental pathways. In functional assays, pNSCs self-renewed for multiple generations and differentiated into functional neurons capable of forming synapses.
This wasn’t just a statistical fluke or artifact. The evidence was clear, reproducible, and overwhelming.
“This was the longest-running project in my career,” said Schöler. “We started with a failure, trying to replicate a now-discredited study. But in that failure, we found something completely new and unexpected. That’s the beauty of science.”
Not Neural Crest, Not Mesenchymal—Something New Entirely
One of the first objections to the pNSC findings was whether these cells were just neural crest-derived stem cells, a known but limited stem cell population found outside the CNS. Neural crest stem cells do exist in peripheral tissues, but their neurogenic capacity and self-renewal potential are notably constrained. The pNSCs, on the other hand, exhibit a long-term ability to self-renew and a more complete range of neurogenic capabilities—making them fundamentally different in behavior and potential.
Their genetic and epigenetic blueprints confirm it. These aren’t misidentified crest cells or mesenchymal imposters. These are legitimate neural stem cells, existing where no one expected them.
A Glimpse Into the Future: Human Translation and Ethical Promise
Though the research has so far been limited to mice, the implications for human health are profound. Current and upcoming studies are already investigating whether pNSCs exist in human tissues—and if so, how they can be harvested, cultured, and applied in therapeutic contexts.
If successful, this would mark a major leap forward in regenerative medicine. Unlike many stem cell therapies that require immunosuppression, pNSCs harvested from a patient’s own body would minimize the risk of rejection. Moreover, their accessibility could democratize treatments for neurological disorders, moving beyond the ethical and logistical barriers posed by embryonic and fetal stem cells.
Conclusion: The Peripheral Revolution in Neuroscience
The discovery of peripheral neural stem cells is more than a footnote in a biology textbook—it’s a seismic shift in how we understand our own biology. It challenges deep-seated assumptions about the nervous system, expands the known geography of neurogenic potential, and offers a promising new path for treating diseases long deemed incurable.
Hans Schöler and Dong Han’s persistence in the face of a failed replication attempt reflects the unpredictable, often poetic nature of science—where one closed door can reveal an entirely new corridor of discovery.
As research continues to unfold, the world waits with anticipation. If these remarkable cells can indeed be found and harnessed in humans, the age of personalized, regenerative neurological therapy may be closer than we think. What was once dismissed as scientific fantasy might soon become standard medical practice—thanks to a mistake, a second look, and the ever-curious eyes of science.
Reference: Dong Han et al, Multipotent neural stem cells originating from neuroepithelium exist outside the mouse central nervous system, Nature Cell Biology (2025). DOI: 10.1038/s41556-025-01641-w