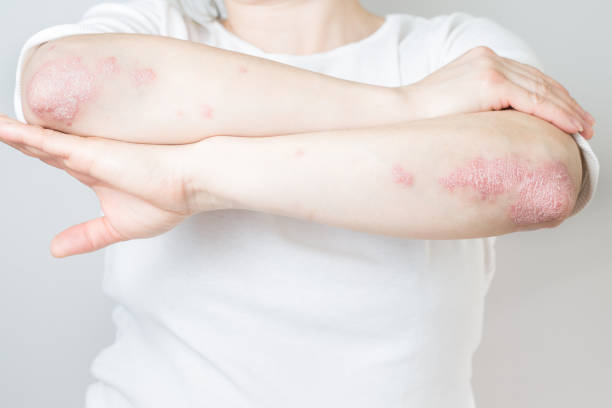
Cancer risk is the shadow that follows every long-term immune-modifying therapy. For people living with moderate to severe psoriasis, biologic drugs can return normalcy to life – restoring clear skin, relieving pain, and protecting joints – but they do so by altering the immune system that ordinarily polices early cancer cells. Whether these medicines subtly shift cancer risk after years of use is one of the biggest unanswered questions in dermatology. Until recently, evidence had been fragmentary, short-term, or too small to be decisive.
A research team from Copenhagen University Hospital–Herlev and Gentofte has now mined nationwide registry data to simulate what a long, head-to-head clinical trial might have revealed if thousands of patients had been directly assigned to competing psoriasis biologics and tracked for half a decade. Their analysis, published in JAMA Dermatology, offers a cautiously worded but attention-grabbing signal: patients treated with ustekinumab may experience a lower five-year risk of developing a first cancer than those taking adalimumab — at least under certain modeling assumptions.
Reconstructing a Trial From the Real World
Because cancer often develops slowly, the true long-term safety profile of biologics cannot be resolved in short randomized trials. The Danish group turned instead to rich national registries, identifying 2,878 adults with psoriasis who began biologic therapy and had no prior history of cancer. Of these, 2,001 started adalimumab, 286 started secukinumab, and 591 started ustekinumab. Rather than simply comparing crude rates, the researchers structured the analysis to emulate a randomized trial: applying lags to account for cancer latency, adjusting for age, sex, and psoriatic arthritis, and estimating standardized five-year risks by modeling survival curves with Cox regression and the G-formula.
Lags were essential. Without a delay period after drug start and drug stop, a cancer that had already been silently growing might be misattributed to a biologic begun only weeks earlier — a classic case of reverse causation. In the main 12-month lag analysis, the differences between drugs were not statistically clear; it remained plausible that no difference existed. But in a sensitivity analysis requiring a 24-month lag — a more conservative assumption about the biology of cancer initiation — a striking estimate emerged: ustekinumab users showed a 56% lower risk of first cancer over five years compared with adalimumab, with confidence intervals that did not cross neutrality.
What the Signal Means — and What It Does Not
The authors are careful not to overclaim. This was not a randomized trial, and registry data, however detailed, cannot capture every relevant nuance of patient biology and clinician decision-making. Clinical “channeling” — the tendency of physicians to choose one biologic over another based on subtle features of disease severity, comorbidity, or perceived frailty — may have influenced the mix of patients in ways that were only partly measurable. For example, psoriatic arthritis was more common among secukinumab users than among ustekinumab users, a difference that may carry hidden implications for cancer risk or immune activation. Even with statistical adjustments, unmeasured confounding may remain.
The investigators also could not dissect risks for specific cancers. It is possible that cancers with very different biology — lymphomas, lung cancers, gastrointestinal tumors, solid vs. hematologic malignancies — might respond differently to biologic immune modulation. A global “any cancer” endpoint smooths over such distinctions.
For these reasons, the authors emphasize interpretation in comparative rather than causal terms. The data suggest a risk gradient; they do not prove that one drug lowers cancer risk, nor that another raises it. Still, the direction and magnitude of the ustekinumab signal — especially under longer latency assumptions — are difficult to ignore.
Why This Matters for Patients and Clinicians
Psoriasis is not a short illness, and biologic therapies are often lifelong. Even a modest shift in long-term cancer risk could have profound implications when multiplied across decades of use and millions of patients. At the same time, undertreatment also carries risk: uncontrolled inflammation is itself linked to systemic illness, including cardiovascular disease and perhaps malignancy through chronic immune stimulation. Clinicians must constantly balance symptom relief, joint protection, cardiovascular risk, quality of life, affordability, and now potentially divergent cancer trajectories.
The Danish analysis offers a glimmer of reassurance for ustekinumab and, to a lesser extent, for IL-17 inhibitors such as secukinumab, whose relatively low observed incidence is framed as “clinically reassuring.” It does not indict adalimumab but suggests the necessity of longer, larger, and more granular surveillance.
The Road Ahead
Biologics have transformed the expectations of what living with psoriasis can mean. They are among medicine’s success stories — targeted, potent, and often life-changing. But success brings responsibility: a therapy used for 10, 20, or 30 years must be scrutinized over similar timelines. The Danish registry study demonstrates that it is now possible to approximate the insight of long-term randomized trials by careful use of real-world data and principled causal modeling.
Future research will need to push further — resolving risk by cancer type, by duration, by dose, by age at initiation, and by combination with other immune-active drugs. If the early signal for ustekinumab holds, it could reshape prescribing patterns, trial design, and regulatory guidance. If it softens with further data, the act of rigorous surveillance will still have achieved its aim: protecting patients against dangers that emerge only with time.
For now, the study provides neither alarm nor final answers, but something subtler and arguably more important — a direction. In the long arc of biologic therapy, cancer risk is not a peripheral question. It is central. And with each new dataset capable of spanning years instead of months, dermatology moves closer to knowing not only how to control disease today, but how to preserve health decades from now.
More information: Christopher Willy Schwarz et al, Comparative Cancer Risk in Patients With Psoriasis Treated With Adalimumab, Secukinumab, or Ustekinumab, JAMA Dermatology (2025). DOI: 10.1001/jamadermatol.2025.3928