Schizophrenia has long been one of the most mysterious and misunderstood psychiatric conditions. It is a chronic disorder that can profoundly alter the way people perceive and interpret reality, often manifesting through hallucinations, delusions, disorganized thinking, and unusual patterns of movement or speech. For those who live with it, schizophrenia can be debilitating, and its expression is far from uniform—each individual’s experience is uniquely shaped by biology, environment, and personal history.
For decades, scientists have sought to uncover the neurobiological roots of this complex disorder. If we could understand what is happening in the brain when schizophrenia emerges, we might one day prevent it from developing, or at least create more precise and effective treatments. Yet the journey toward such understanding has been anything but straightforward.
Neuroimaging—using tools like MRI and PET scans to study the brain—has revealed glimpses of what might be happening. However, many results have been inconsistent or contradictory. Researchers struggled to define a clear picture of which brain regions were altered, when changes occurred, and how they linked to symptoms. Now, new work from researchers at Taipei Medical University offers a promising step toward clarity.
A New Approach to a Difficult Question
In a study recently published in Nature Mental Health, Matteo Martino, Paola Magioncalda, and their colleague Abhishek Yadav attempted to cut through the noise. Instead of adding one more neuroimaging study to an already crowded field, they turned to a broader perspective: an umbrella review.
This approach involved gathering and analyzing dozens of existing meta-analyses, each of which had already summarized hundreds of neuroimaging studies on schizophrenia. By combining this vast body of research and mapping the data onto a common framework, the team hoped to identify patterns that were both consistent and biologically meaningful.
As Martino and Magioncalda explained, a first step toward progress is to reliably map the brain changes that accompany schizophrenia. Only by knowing where, when, and how these changes occur can researchers begin to understand the mechanisms that give rise to symptoms such as hallucinations, delusions, and cognitive decline.
Tracing the Brain’s Changing Landscape
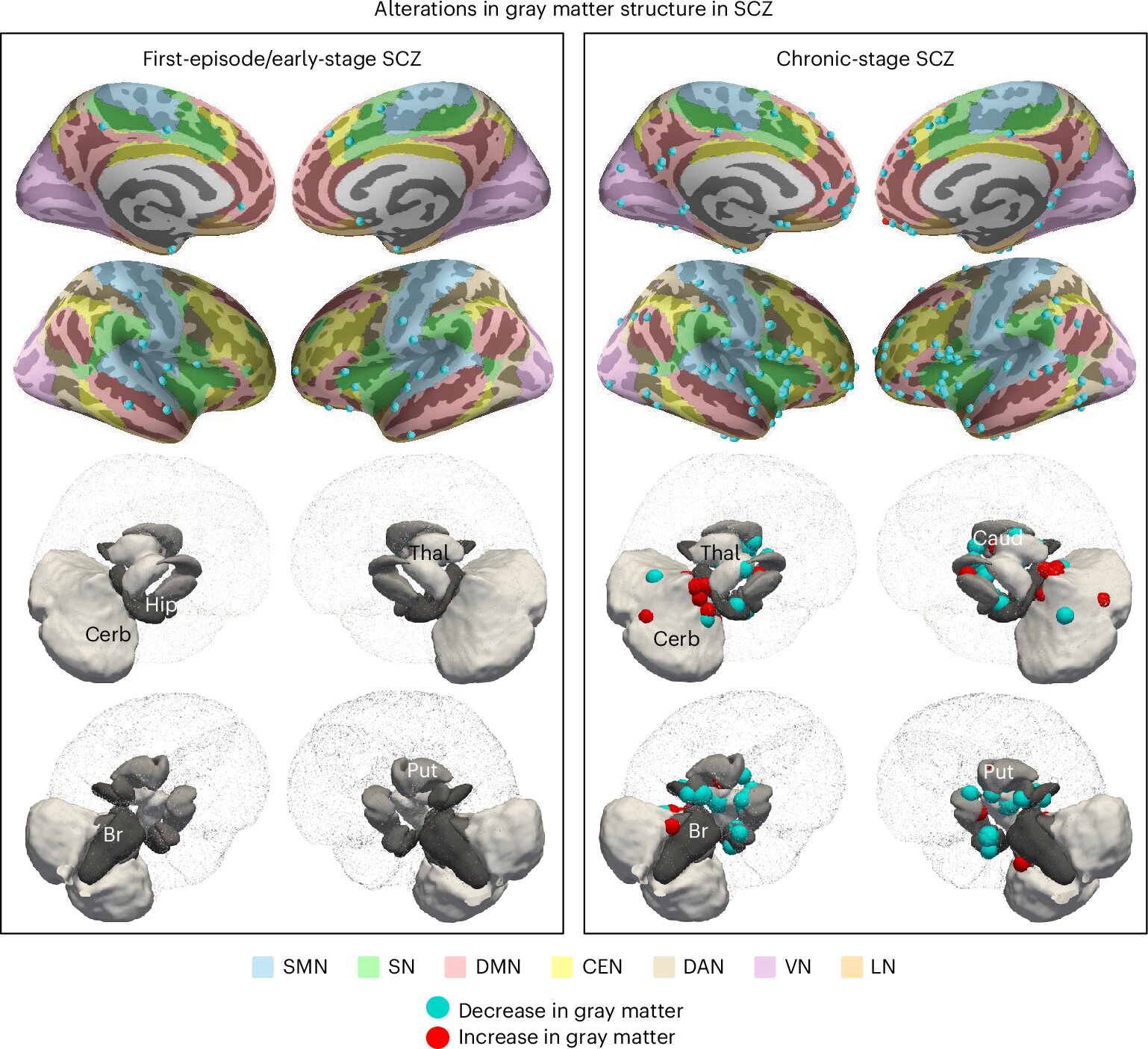
The results of the umbrella review provided a detailed map of brain alterations that appear across different stages of schizophrenia, from the earliest warning signs to chronic illness. These findings suggest that schizophrenia is not static but evolves over time, spreading through the brain in recognizable patterns.
In the prodromal stage—the early period before full psychosis emerges—the most consistent alterations appear in midline regions such as the medial prefrontal cortex. This area plays a key role in self-awareness and decision-making, and its early vulnerability may reflect developmental risk factors that shape the brain long before symptoms are visible.
As the illness progresses into the early psychotic stage, changes extend outward. Gray matter loss becomes evident in opercular regions such as the insula and the superior temporal gyrus, while white matter near the brain’s ventricles also shows disruption. At the same time, dysfunction emerges in the brain’s default-mode network, a system active during rest and self-referential thought. Together, these alterations suggest that both structural and functional changes in the brain converge to destabilize perception and thought.
By the chronic stage of schizophrenia, the picture becomes more severe and widespread. Damage increasingly affects the thalamus and prefrontal cortex, regions essential for processing sensory information and supporting high-level cognition. This could help explain the gradual decline in cognitive abilities that many patients experience over time.
Linking Brain Changes to Symptoms
One of the most compelling aspects of this new model is its ability to connect specific brain alterations with hallmark symptoms of schizophrenia.
For instance, damage to the superior temporal gyrus—a region that houses the auditory cortex—was consistently associated with auditory hallucinations, the perception of voices that are not actually present. Similarly, dysfunction of the default-mode network was linked to delusions, the rigidly held false beliefs that can dominate the thoughts of patients.
These links offer more than just explanations; they suggest that symptoms of schizophrenia may arise from distinct neural pathways, rather than from a single underlying disruption. This insight could one day allow for more targeted treatments that address specific symptoms rather than the disorder as a whole.
The Role of Immune Mechanisms
One intriguing hypothesis raised by the researchers involves the role of immune processes in driving brain changes. Because many structural alterations occur near cerebrospinal fluid, the fluid that bathes the brain and spinal cord, the team speculates that immune-related factors may spread through this fluid, damaging nearby tissue. If true, this could open an entirely new avenue of research into how inflammation and immunity interact with brain development and psychiatric illness.
Another key hypothesis concerns sensory cortices—the regions that process visual, auditory, and tactile information. If these areas sustain structural deficits, they may give rise to specific types of hallucinations depending on which sense is affected. This framework positions psychosis not as a single mystery but as a set of symptom pathways rooted in distinct brain vulnerabilities.
Toward a Biological Model of Schizophrenia
The umbrella review represents more than a summary of past research—it marks a step toward a biological model of schizophrenia that is grounded in the most consistent evidence to date. By integrating structural, functional, and symptomatic data, Martino, Magioncalda, and Yadav offer a roadmap for understanding how schizophrenia develops and evolves in the brain.
Such a model does not yet explain everything. The causes of schizophrenia remain uncertain, and factors such as genetics, early brain development, and environment all likely play essential roles. But the ability to identify reliable brain alterations and map their progression is a powerful step toward building therapies that address the disorder at its roots.
Implications for the Future
The significance of this work extends beyond schizophrenia alone. The researchers are already exploring overlaps between schizophrenia, major depressive disorder, and bipolar disorder, with the aim of disentangling what these conditions share and what makes each unique. By doing so, they hope to contribute to an integrated framework for understanding severe mental illness as a whole.
In practical terms, this means future treatments could become far more precise. Instead of broadly targeting symptoms with medications that have significant side effects, doctors might one day use neuroimaging-guided interventions tailored to the biological stage or symptom pathway of each patient.
Equally important, advancing our understanding of schizophrenia’s biology can reduce stigma. Too often, people with schizophrenia are misunderstood or marginalized. Showing that symptoms arise from identifiable brain alterations—not from weakness or personal failing—helps reframe the condition as a medical challenge, not a moral one.
A Continuing Journey
The work of Martino, Magioncalda, and Yadav represents progress, but it is not the end of the story. New empirical findings, refined neuroimaging methods, and deeper explorations into immune processes and sensory systems will continue to reshape our understanding.
Schizophrenia remains one of the great frontiers of psychiatry, a condition that challenges both scientific inquiry and human empathy. But with each step forward—each clearer map of the brain, each new model of how symptoms emerge—we move closer to a future where treatment is not just about managing illness, but about restoring balance, function, and hope.
Conclusion: Toward Clarity and Compassion
Schizophrenia is a disorder that can shatter the fabric of reality for those who experience it, yet it also reveals the resilience of the human mind. Science is now beginning to untangle its mysteries, tracing the shifting patterns of brain alterations that underlie hallucinations, delusions, and cognitive decline.
The new biological model proposed by researchers in Taipei does more than illuminate neural pathways; it builds a foundation for compassion and progress. By understanding schizophrenia as a dynamic brain-based condition, we take a vital step toward developing therapies that heal and toward a society that supports, rather than stigmatizes, those who live with it.
In the end, the quest to understand schizophrenia is not only a scientific journey but also a human one—a reminder that to map the brain is also to honor the lives and struggles of those whose realities it shapes.
More information: Paola Magioncalda et al, An umbrella review of neuroimaging studies and conceptual framework linking pathophysiology and psychopathology in schizophrenia, Nature Mental Health (2025). DOI: 10.1038/s44220-025-00493-5.