Imagine your brain as a bustling city, with different neighborhoods working together or competing for attention. In one corner, you have the default mode network, a region responsible for quiet reflection, daydreaming, and self-referential thoughts. On the other hand, there’s the dorsal attention network, a system that kicks into action when you focus on specific tasks, like reading, problem-solving, or driving. These two networks often play a game of tag—when one is busy, the other rests. But what happens when the lines blur and these two systems can’t seem to separate their roles?
Recent research out of Université Savoie Mont Blanc, Radboud University, and the University of Oxford’s Wellcome Center for Integrative Neuroimaging suggests that this very blurring could hold the key to understanding Alzheimer’s disease in ways we never have before. In a study published in NeuroImage, these researchers revealed how disruptions in the interaction between these two brain networks could be a critical marker of Alzheimer’s-related cognitive decline—potentially offering a fresh, non-invasive biomarker that could help identify the disease earlier and more accurately.
The Brain’s Power Struggle: Default Mode vs. Dorsal Attention
Think of the default mode network as the brain’s “rest mode.” When you’re not actively focused on the world around you—say, when you’re reflecting on a recent trip or imagining future events—this network is working in the background. The dorsal attention network, on the other hand, springs to life when you need to focus. If you’re trying to solve a math problem or avoid getting distracted while reading, it’s this network that ensures you stay on task.
These networks are meant to work in opposition. When one is active, the other tends to shut down. But in Alzheimer’s disease, something goes awry. The default mode and dorsal attention networks start to lose their separation. They start to overlap, making it harder for the brain to keep attention sharp and thoughts organized. It’s a subtle change that can significantly impact cognitive function over time, leading to the memory loss and confusion that defines Alzheimer’s.
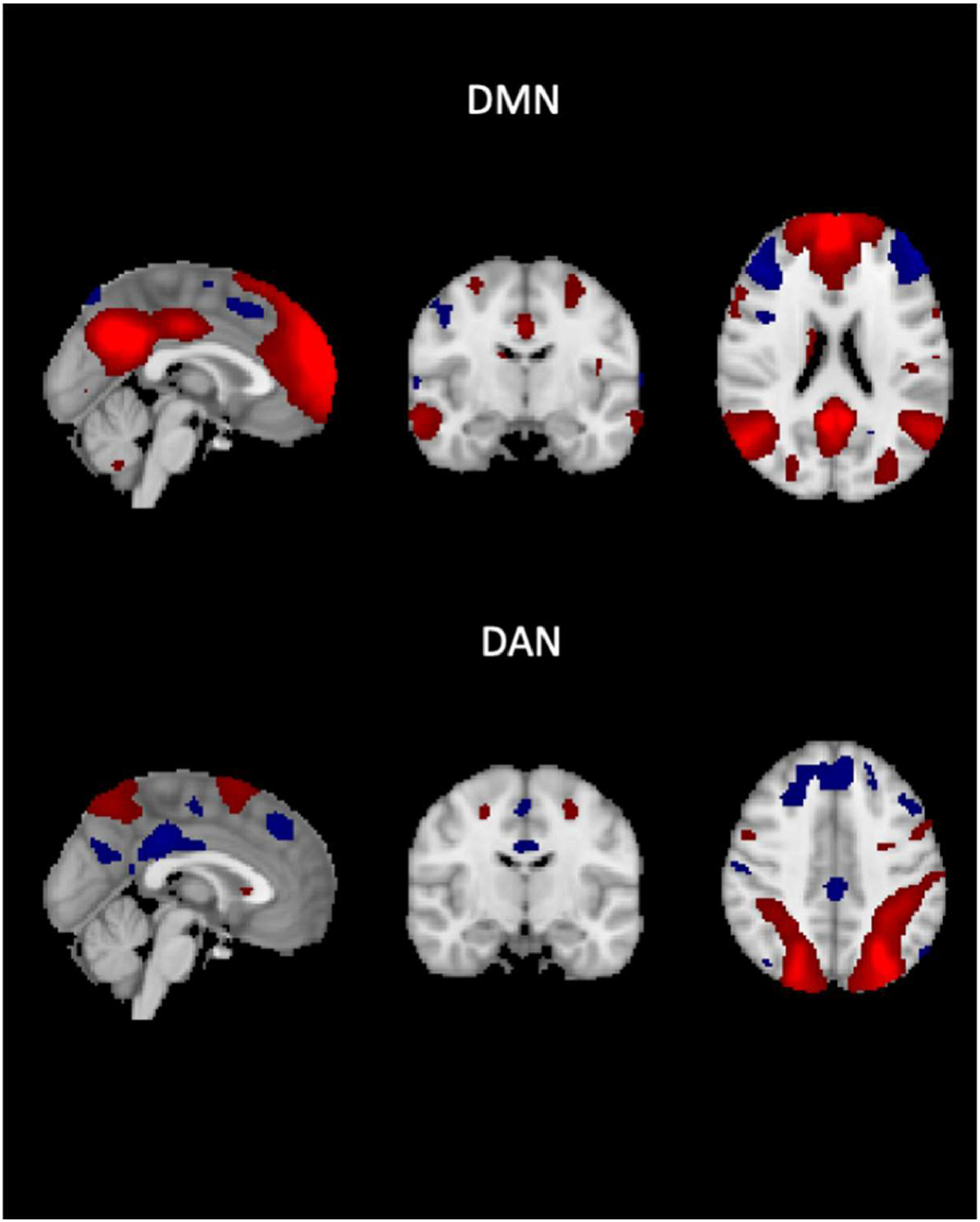
In their groundbreaking study, the researchers used resting-state functional MRI (fMRI) to track how these two networks interact in the brain. What they found was striking: a loss of the usual “on/off” relationship between the default mode and dorsal attention networks in participants with high amyloid load and cognitive impairment. This dysfunction in network separation could signal the beginning of cognitive decline, long before more visible symptoms like memory loss appear.
The Science Behind the Discovery
To dive into this, the team analyzed data from 182 participants in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort. They looked at a combination of fMRI scans, cognitive evaluations, and imaging data that measured amyloid and tau protein deposits—two hallmarks of Alzheimer’s pathology. What they found was that participants with more amyloid in their brains also had a weaker distinction between the default mode and dorsal attention networks.
In essence, the clearer the separation between these two networks, the better participants scored on cognitive tests. As the networks lost their separation, cognitive function suffered. And perhaps even more compelling, this loss of network separation didn’t seem to be entirely linked to the well-known tau protein accumulation that is often blamed for Alzheimer’s-related damage. Instead, the pattern appeared to be an independent marker of cognitive decline.
This discovery suggests that something deeper is at play—a multifaceted mechanism of decline that isn’t solely driven by tau or amyloid buildup. Rather, this altered brain network connectivity could be a window into how Alzheimer’s disease affects the brain on a more fundamental level, offering clues to potential treatments or earlier interventions.
The Implications for Diagnosis and Treatment
For years, researchers have struggled to find a definitive, easily measurable biomarker for Alzheimer’s disease. While amyloid and tau proteins are often used as indicators, they don’t always align perfectly with cognitive decline. This new finding, however, could be a game-changer. By focusing on the relationship between the default mode and dorsal attention networks, scientists might have discovered a more reliable way to track cognitive decline as it happens—not just a snapshot of disease progression, but a real-time glimpse into how the brain is functioning.
What’s exciting is that this marker isn’t dependent on tau pathology or the typical “cognitive reserve” offered by education or lifestyle factors. Previous research has often suggested that individuals with more education or higher cognitive reserve may show different patterns of brain decline. But this new network-based measure cuts through that complexity, providing a cleaner, more direct link to cognitive decline that is independent of these other factors.
But the implications go beyond diagnosis. If we can track these network changes early on, it might allow for more personalized treatment approaches. Doctors could focus on stabilizing network activity or even intervene to prevent the decline before it becomes too severe. This would be a major leap forward in Alzheimer’s research, where treatments to stop or even reverse the disease remain elusive.
Looking Ahead: What Comes Next?
The researchers behind this study have laid a fascinating foundation for future research. The next steps will involve testing whether this altered brain network interaction actually occurs before the buildup of amyloid and tau proteins. It could also be a signal that tracks not just the progression of the disease but the broader, multifactorial causes of cognitive decline, such as vascular disease, stress, or sleep loss—all of which can influence brain function.
The researchers also point to the need for longitudinal studies—research that tracks patients over a long period—to determine whether these network changes predict the onset of Alzheimer’s before the classic symptoms appear. If confirmed, this could offer an early detection method that doesn’t require invasive tests or complex procedures. The potential to catch Alzheimer’s before the cognitive damage becomes irreversible is one of the most exciting prospects in neuroscience today.
Ultimately, this discovery brings us a step closer to understanding Alzheimer’s disease not just as a single event, but as a complex, evolving process that affects the brain in ways we are only beginning to appreciate. With more research, we may soon have new ways to monitor, predict, and perhaps even slow down the course of this devastating condition.
Why This Matters
The most significant takeaway from this study is that we may be on the verge of a breakthrough in Alzheimer’s diagnosis and treatment. While much of the focus has been on amyloid and tau deposits, this new research suggests that the disruption of brain networks—particularly the relationship between the default mode and dorsal attention networks—could be just as crucial a marker of disease progression.
The ability to track Alzheimer’s through network behavior, rather than just protein buildup, opens new doors for early diagnosis and personalized treatment. This is a profound shift in how we understand and potentially combat a disease that affects millions of people worldwide.
In the end, the true power of this discovery lies in its potential to transform the way we detect and treat Alzheimer’s. By honing in on the intricate dance between brain networks, we might one day be able to catch the disease early enough to slow its devastating impact. That possibility brings new hope to the fight against one of the most challenging diseases of our time.
More information: Diego-Martin Lombardo et al, The intrinsic connectivity between the default mode and dorsal attention networks is an independent fMRI biomarker of Alzheimer’s disease pathology burden, NeuroImage (2025). DOI: 10.1016/j.neuroimage.2025.121509