For centuries, red blood cells have been seen as the silent laborers of the bloodstream—tirelessly ferrying oxygen from lungs to tissues, their biconcave shapes gliding effortlessly through capillaries. They’ve been drawn in textbooks, observed under microscopes, and studied in medical schools around the world. Yet, for all that familiarity, a major piece of their puzzle was still missing.
Now, in a groundbreaking study from the University of Pennsylvania, scientists have revealed a startling truth: red blood cells don’t just passively get trapped in blood clots—they help build them. Actively. Powerfully. Purposefully.
This discovery turns one of biology’s most fundamental understandings on its head, and it could change how we treat bleeding disorders and strokes.
The Unexpected Architects of Clot Contraction
“We always thought platelets were doing all the work,” says Dr. Rustem Litvinov, a senior researcher at the Perelman School of Medicine and one of the study’s lead authors. “But it turns out, red blood cells are not just bystanders—they’re builders.”
This new insight, published in Blood Advances, reveals that red blood cells actually play a hands-on role in clot contraction, the process by which a newly formed clot shrinks and stabilizes. Traditionally, this job was attributed solely to platelets, the small disc-shaped fragments that rush to plug wounds and tighten the fibrin mesh, like drawstrings pulling a net closed.
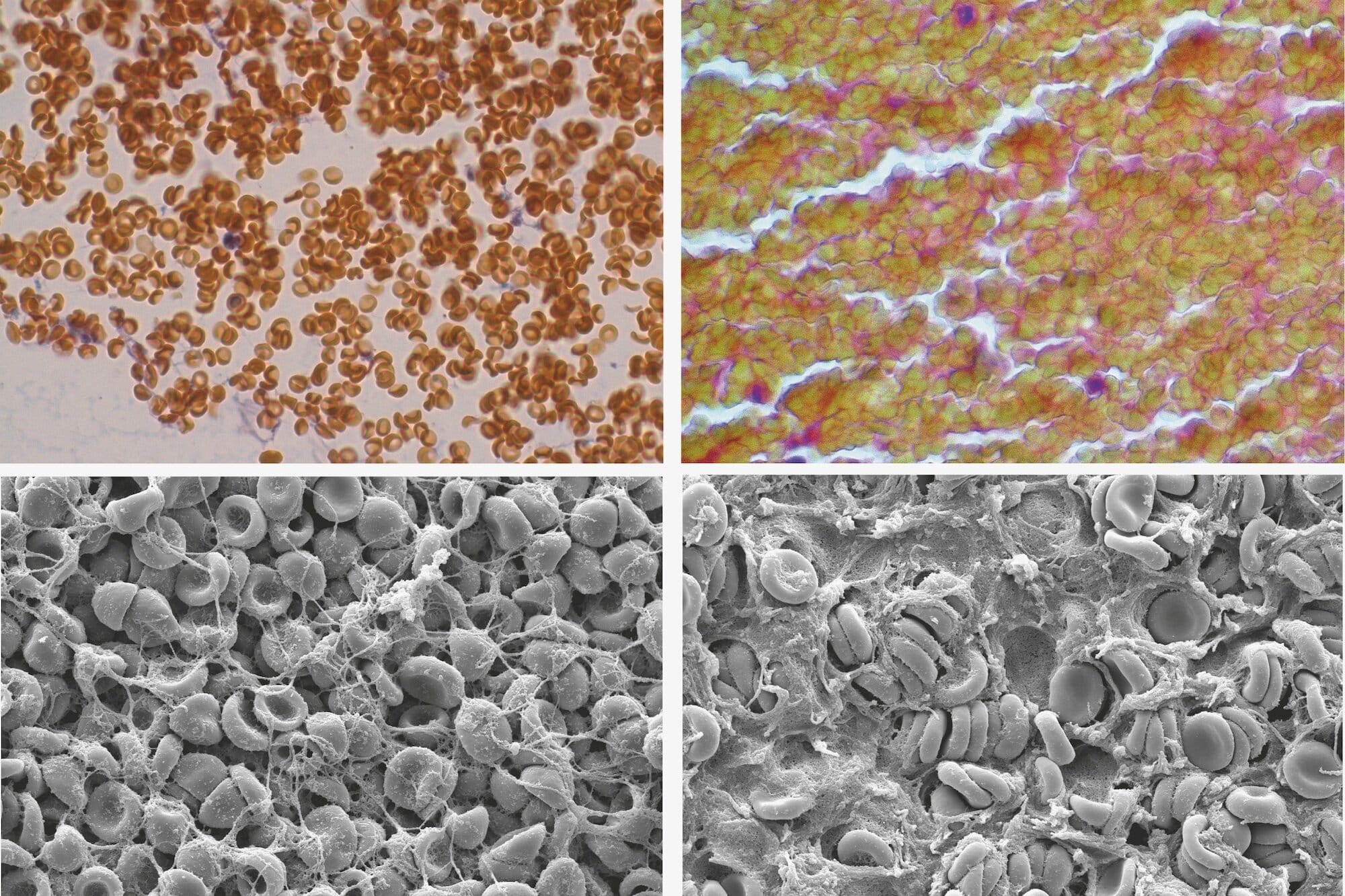
But when researchers formed clots without platelets, expecting them to remain loose and unstable, they were stunned to find the clots still contracted—shrinking by over 20%. Something else was at work.
A Moment of Scientific Serendipity
That something, as it turned out, was hiding in plain sight.
Dr. John Weisel, co-author of the study and professor of Cell and Developmental Biology at Penn, recalls the moment it became clear: “We designed an experiment expecting to prove that without platelets, clots can’t contract. But they did. It was like discovering an extra set of hands in the dark.”
The team repeated the experiment, this time using normal blood treated with chemicals that block platelet activity. Again, the clots contracted. This unexpected finding set off a cascade of new questions and a thrilling investigative journey.
“It forced us to rethink what we thought we knew,” says Litvinov. “If red blood cells weren’t just getting stuck in the fibrin net, what were they doing?”
Modeling the Invisible Mechanics
To answer that, the researchers turned to Dr. Prashant Purohit, a professor of mechanical engineering and applied mechanics at Penn Engineering. Purohit is an expert in the strange, squishy world of soft materials—gels, colloids, and now, blood clots.
Using his background in physics and engineering, Purohit built a mathematical model to explore how red blood cells might physically influence the structure of a clot. What he found was something unexpected: a process called osmotic depletion.
In simple terms, osmotic depletion is like the pressure imbalance you see when you put a raisin in water—the water rushes in to balance the concentration of solutes. But here, it’s turned inside out.
When red blood cells get packed tightly inside a forming clot, the proteins and molecules in the surrounding fluid are forced out of the narrow spaces between them. That creates a pressure imbalance: the concentration of solutes becomes higher outside the cluster of cells than inside, which causes water to flow out and forces the red blood cells closer together.
“It’s almost like an invisible squeeze from the outside,” Purohit explains. “This causes red blood cells to clump together, exerting mechanical forces that help the clot contract and strengthen.”
Rewriting the Rules of Clotting
For decades, biology textbooks have painted a familiar picture of blood clotting: first comes injury, then platelets arrive and stick to the damaged vessel walls. A fibrin net forms to trap cells, and platelets pull on the net to contract and stabilize the clot. Red blood cells, while present in this process, were viewed as little more than filler material—cork stuffed into the plug.
But this new research tells a different story.
“Red blood cells are not just passengers,” says Purohit. “They actively engage in reshaping the clot.”
Their contribution, though subtle, is significant. Once the fibrin network captures them, the osmotic depletion effect triggers a cascade of cellular rearrangements, which not only compacts the clot but may also increase its durability and resistance to shear forces from flowing blood.
“This process is helping the body form a tighter, more resilient barrier at the site of injury,” Purohit adds. “It’s evolutionarily elegant.”
Validating the Vision: From Model to Microscopy
Of course, a mathematical model is only as good as its experimental validation. That’s where Dr. Alina Peshkova, first author of the study, came in. A postdoctoral researcher in Pharmacology at PSOM, Peshkova designed meticulous lab experiments to test Purohit’s predictions.
First, she modified clots to eliminate the possibility of molecular “bridging”—the theory that red blood cells might stick together due to adhesive surface proteins. Even without bridging molecules, the clots still contracted.
Then, she engineered a clotting environment in which osmotic depletion forces couldn’t develop. In that setting, the red blood cells failed to cause contraction.
“The results were crystal clear,” says Peshkova. “The contraction was happening because of osmotic depletion, not adhesion. It’s rare in biology to see such strong agreement between theory and experiment.”
This beautiful convergence of physics, engineering, and biology gives scientists a new lens through which to view one of the body’s most essential systems.
Implications for Stroke, Bleeding Disorders, and More
Understanding how clots contract isn’t just an academic exercise. It could have profound medical implications—especially for patients suffering from clotting disorders, strokes, or embolisms.
In conditions like thrombocytopenia, where patients have dangerously low platelet counts, doctors often struggle to control bleeding. The new discovery that red blood cells can drive clot contraction without platelets opens potential avenues for therapy.
“Maybe we can design drugs or treatments that enhance this red blood cell-driven contraction,” says Litvinov. “That could help patients who can’t produce enough platelets.”
On the flip side, knowing how clots contract might also help doctors prevent dangerous over-contraction. In some cases, clots shrink too tightly, break apart, and travel through the bloodstream—causing strokes or pulmonary embolisms. Targeting the mechanical processes behind red blood cell clustering might allow for more precise control of clot behavior.
“This is a whole new toolkit,” says Purohit. “We’re not just looking at clotting through the lens of chemistry anymore. We’re now looking at it through the lens of physics and mechanics.”
Rethinking the Building Blocks of Blood
One of the most remarkable aspects of this study is how it challenges long-standing assumptions. Red blood cells have been known since the days of early microscopy. Yet here we are, in the 21st century, learning something entirely new about them.
“It’s humbling,” says Litvinov. “We’ve studied red blood cells for hundreds of years. But the body is full of secrets. Sometimes you have to ask the question that no one else thought to ask.”
That spirit of curiosity is at the heart of this discovery. It’s a reminder that science is not a set of static facts, but a living process—one that evolves, surprises, and delights.
A New Chapter in Clotting Research
This breakthrough is more than just a single paper. It represents a turning point in how we study blood, wounds, and healing.
Researchers are already exploring follow-up questions: Do certain diseases affect the osmotic depletion process? Do red blood cells behave differently in people with anemia or sickle cell disease? Can we engineer artificial blood that mimics these mechanical properties?
By combining physics with biology, this research paves the way for a more holistic understanding of human health.
“Ultimately, the body is a masterpiece of soft matter engineering,” says Purohit. “And we’re only just beginning to understand the blueprints.”
More information: Alina D. Peshkova et al, Red blood cell aggregation within a blood clot causes platelet-independent clot shrinkage, Blood Advances (2025). DOI: 10.1182/bloodadvances.2024015533