Why do we feel hungry? Why do some people struggle to control appetite while others seem naturally balanced in their eating? These questions may sound simple, but they cut to the heart of one of humanity’s most urgent health challenges: obesity and metabolic disease. Recently, researchers at Leipzig University and Charité—Universitätsmedizin Berlin uncovered a crucial piece of this puzzle. Their study revealed how a little-known protein acts as a gatekeeper for one of the brain’s most important hunger-regulating systems.
This discovery, published in Nature Communications, not only deepens our understanding of how the brain manages appetite but also points toward new ways to treat obesity—an epidemic affecting hundreds of millions of people worldwide. The findings illuminate the hidden dance of proteins within brain cells, showing us how subtle molecular interactions can shape something as profound as the feeling of hunger.
The Brain’s Master Regulator: MC4R
At the center of this story lies a receptor in the brain known as MC4R—the melanocortin-4 receptor. This receptor acts like a molecular switch for appetite. When activated, MC4R tells the brain that we are satisfied, helping regulate how much food we eat and how much energy we store. Mutations in MC4R are among the most common genetic causes of severe obesity, underscoring just how vital this receptor is to our survival and health.
MC4R is activated by a hormone called MSH (melanocyte-stimulating hormone). When MSH binds to the receptor, it triggers signals that suppress hunger. For years, scientists have been studying MC4R, not only to understand its basic biology but also to identify drugs that might help people whose weight-control systems are disrupted. In fact, one such drug, setmelanotide, is already in use—it directly activates MC4R, reducing hunger in individuals with rare genetic disorders that impair appetite regulation.
Yet, despite these advances, an important question remained: how is MC4R itself managed inside brain cells? How is it moved, positioned, and controlled so that it can properly signal feelings of fullness? This is where the new discovery comes in.
The Role of a Hidden Helper: MRAP2
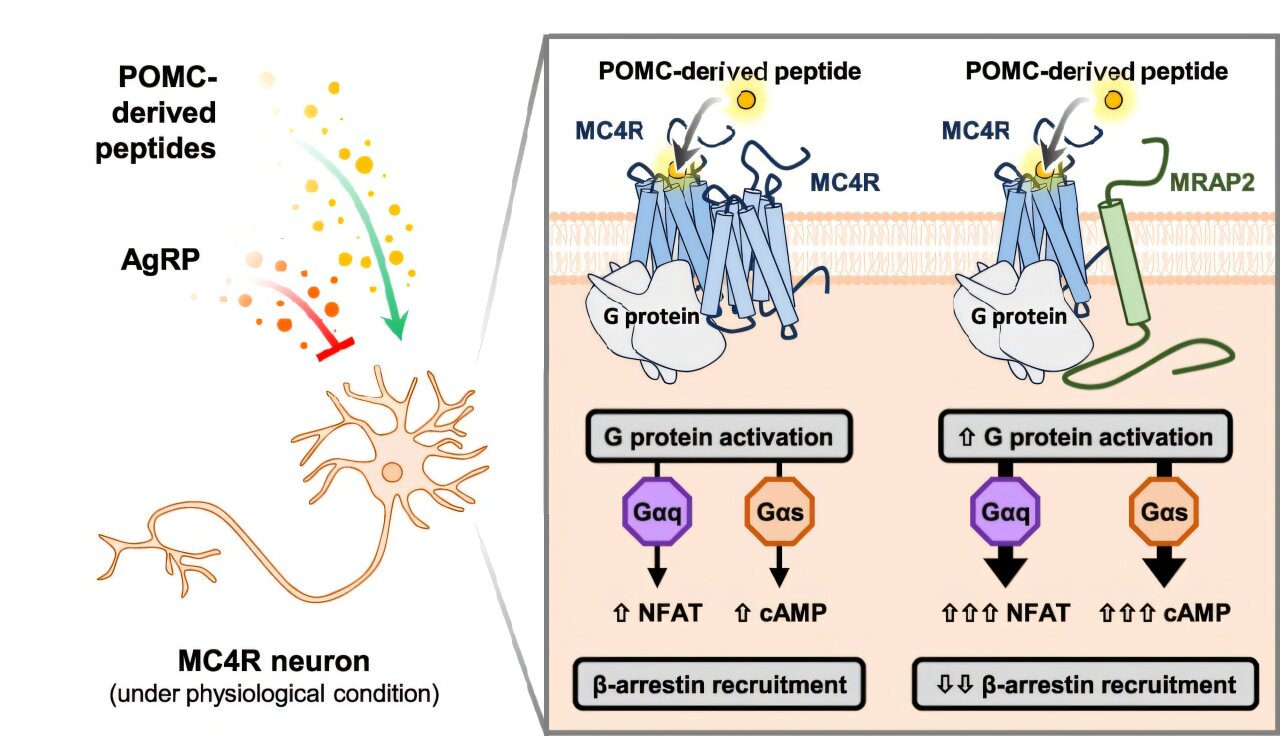
The researchers focused on a protein called MRAP2 (melanocortin 2 receptor accessory protein 2). Until recently, MRAP2 was something of a mystery. Scientists knew it was linked to energy balance and metabolism, but its exact role remained unclear.
Using cutting-edge imaging technologies—fluorescent biosensors, confocal microscopy, and single-cell imaging—the team discovered that MRAP2 plays a vital role in guiding MC4R to the right place within cells. Without MRAP2, much of the MC4R receptor remains trapped inside the cell, unable to reach the cell surface where it needs to be to receive hunger-suppressing signals.
With MRAP2’s help, however, MC4R is transported to the cell surface, like an antenna raised to catch signals. There, it can properly detect hormones such as MSH and translate them into feelings of satiety. In essence, MRAP2 acts as a chaperone or escort, ensuring that MC4R is in the right location to do its job.
A Window Into Cellular Communication
What makes this discovery especially exciting is not only its relevance to obesity but also its demonstration of how complex and beautiful cellular communication really is. Our brain cells are not static—they are dynamic, bustling with proteins that constantly move, interact, and adapt.
By visualizing this process with live-cell imaging, the researchers were able to watch in real time as MRAP2 changed the behavior and location of MC4R. This kind of work bridges physics, biology, and medicine: the molecular choreography inside our cells becomes visible, offering insights that could one day reshape how we treat disease.
Toward New Treatments for Obesity
Obesity is often misunderstood as a matter of willpower, but science tells a different story. For many individuals, appetite and weight are influenced by genetic and molecular factors beyond conscious control. Mutations in MC4R, for instance, directly interfere with the brain’s ability to signal fullness, creating an unrelenting drive to eat.
By uncovering the role of MRAP2 in regulating MC4R, this research opens a potential new therapeutic pathway. If scientists can design drugs that mimic MRAP2’s effect—or enhance its activity—they might improve MC4R’s ability to reach the cell surface and suppress appetite. Such an approach could benefit not only those with rare genetic disorders but also broader populations struggling with obesity and related metabolic diseases like type 2 diabetes.
Professor Annette Beck-Sickinger of Leipzig University, a co-author of the study, emphasized the importance of this finding, noting that understanding receptor transport and availability is just as crucial as knowing how receptors are activated. It is a new layer of regulation—one that had been largely overlooked until now.
Collaboration at the Frontiers of Science
The study was the result of a collaborative effort within the Collaborative Research Center (CRC) 1423, a network devoted to understanding how a large family of receptors known as GPCRs (G protein–coupled receptors) work at the molecular level. Five different projects came together for this study, combining structural biology, imaging, molecular biophysics, and medical expertise.
Dr. Patrick Scheerer from Charité highlighted how earlier structural studies of MC4R—such as mapping its 3D shape when bound to ligands like setmelanotide—paved the way for the new insights into MRAP2’s role. This illustrates how science often advances: one breakthrough lays the foundation for the next, like stepping stones across a river of unknowns.
Another co-lead author, Dr. Paolo Annibale from the University of St Andrews, reflected on the excitement of applying advanced imaging technologies in a context with such direct physiological relevance. Their work shows how physics-inspired tools, like high-resolution microscopy, can transform our understanding of biology.
The Human Story Behind the Molecules
At first glance, proteins like MRAP2 and receptors like MC4R might seem like abstract molecular machines. But behind them lies the human story of hunger, health, and survival. Hunger is one of the most primal experiences we share as a species—it has driven migration, agriculture, and culture itself. When the systems that regulate hunger malfunction, the consequences can be life-altering, leading to obesity, illness, and stigma.
This is why discoveries like this resonate beyond the laboratory. They remind us that biology is not destiny—that by understanding the molecular roots of appetite, we can imagine a future where treatments are tailored to each individual’s needs, where the suffering caused by obesity is reduced, and where hunger itself is better understood and managed.
Looking Ahead
The discovery of MRAP2’s essential role in controlling the melanocortin system is just the beginning. Future research will aim to clarify exactly how MRAP2 interacts with MC4R, whether other proteins are involved in this process, and how these findings can be translated into safe and effective therapies.
For now, the study stands as a milestone—a reminder that even within the most familiar human experiences, like feeling hungry, there are hidden complexities waiting to be uncovered. It shows us that the journey from molecule to medicine is a collective one, requiring not only brilliant science but also international collaboration, creativity, and persistence.
The Deeper Meaning
At its heart, this discovery is not just about proteins and receptors. It is about the human quest to understand ourselves. Hunger is one of the most ancient sensations, shaping the rhythms of our days and the course of civilizations. To uncover the molecular mechanisms behind it is to peel back another layer of what it means to be alive.
The work of the Leipzig and Charité teams gives us hope that knowledge, carefully built and shared, can improve lives. It is a story of curiosity and perseverance—a reminder that within the microscopic machinery of our cells lie answers to some of the most pressing challenges of our time.
More information: Iqra Sohail et al, MRAP2 modifies the signaling and oligomerization state of the melanocortin-4 receptor, Nature Communications (2025). DOI: 10.1038/s41467-025-63988-w