For decades, one of the greatest challenges in cancer treatment has been the problem of “immune cold” tumors—tumors that hide from the immune system, cloaking themselves so well that even the body’s most powerful defenders, T cells and B cells, fail to recognize them. These stealthy cancers often resist chemotherapy and immunotherapy, leaving patients with few effective options. But now, in groundbreaking research from Johns Hopkins All Children’s Hospital, scientists have shown that it may be possible to rewire the immune landscape inside these resistant tumors, transforming them into immune “hot” battlegrounds that the body can finally fight back against.
The study, published in Nature Immunology, uses mouse models of breast, pancreatic, and muscle cancers to reveal how stimulating the right immune pathways not only halts tumor growth but also creates lasting, systemic protection against relapse. The approach doesn’t just attack cancer—it teaches the immune system how to remember it.
Immune Cold Versus Immune Hot
Not all tumors are created equal in how they interact with the immune system. Some are “immune hot,” rich with immune cells and active inflammation. In these tumors, the immune system is engaged and therapies like checkpoint inhibitors often have dramatic effects. But other tumors are “immune cold,” barren landscapes devoid of active immune defense. In these environments, immune cells either fail to enter or are quickly suppressed, leaving cancer cells free to grow and spread.
Patients with immune cold tumors typically face the worst outcomes. They may undergo surgery, radiation, or chemotherapy, but relapse is common. The dream of modern immunotherapy—harnessing the patient’s own immune system to destroy cancer—remains out of reach for many of these patients. The new study offers a potential way forward, a method to flip cold tumors into hot ones.
The Power of Tertiary Lymphoid Structures
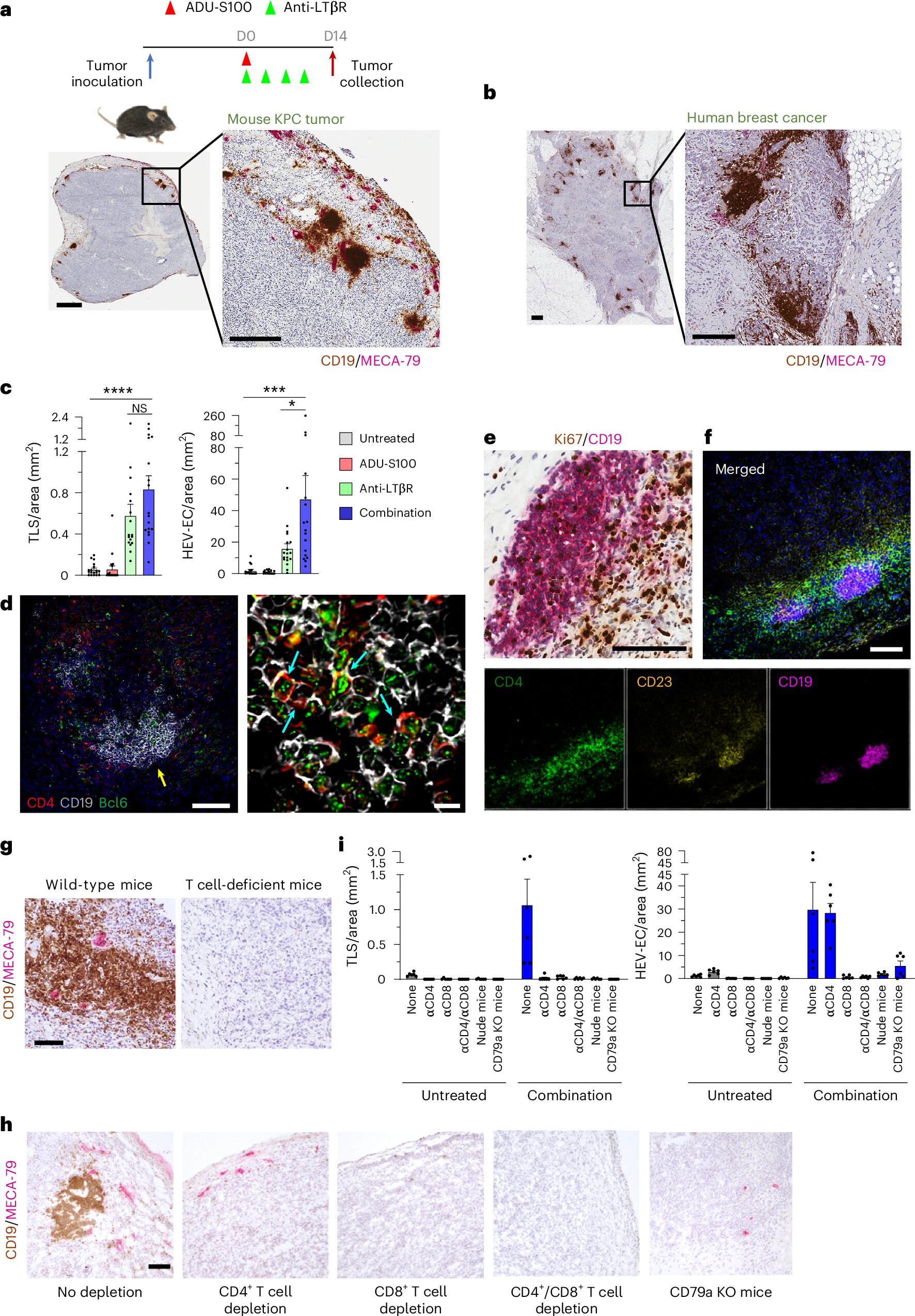
At the heart of this discovery is something called tertiary lymphoid structures, or TLSs. These are clusters of immune cells that resemble mini-lymph nodes forming inside tumors. Like command centers, TLSs recruit, organize, and activate immune responses directly at the site of disease. Their presence strongly correlates with better responses to therapy and longer survival in cancer patients.
But not all tumors naturally develop TLSs. The challenge for scientists has been how to create them where they don’t exist—how to re-engineer an immune desert into a fortified stronghold.
Reverse-Engineering Immunity
The Johns Hopkins team set out with a bold idea: if they could determine the signals required for TLS formation, they might be able to artificially induce them in immune cold tumors. They identified two crucial stimulators of immune activity:
- STING, a protein that alerts the immune system to danger, often triggered by viral DNA or cellular stress.
- LTβR, a receptor important in lymphoid organ development and immune cell organization.
By delivering agonists—molecules that activate these proteins—directly into tumors, the researchers created a spark that transformed the immune environment.
A Tumor Awakens
The results were dramatic. Within days, killer T cells (CD8⁺ T cells) swarmed the tumors, attacking cancer cells with renewed vigor. At the same time, specialized blood vessels called high endothelial venules formed inside the tumors. These vessels act like gateways, allowing large numbers of lymphocytes to flood in. For the first time, tumors that had been cold and barren were teeming with immune activity.
Inside the newly formed TLSs, B cells organized themselves into germinal centers, specialized hubs where they matured into plasma cells capable of secreting antibodies. These antibodies targeted the tumors directly, marking them for destruction. Even more impressively, the B cells generated long-lived memory cells, ensuring that the immune system would remember the cancer if it ever tried to return.
The researchers also found evidence of systemic immunity. Plasma cells migrated into the bone marrow, a safe harbor where they can survive for years, continuing to produce protective antibodies. This suggested that the treatment not only controlled tumors in the short term but also built a shield against recurrence.
Building Balance: T Cells, B Cells, and Lasting Immunity
The therapy did more than boost one type of immune cell. It created balance. Helper T cells (CD4⁺) increased in number, providing essential support to both killer T cells and B cells. Memory T cells emerged, capable of rapid response upon re-exposure to the cancer. In this way, the immune system’s two great arms—humoral immunity (driven by antibodies) and cellular immunity (driven by T cells)—worked together, amplifying and sustaining each other.
This was not just an immune flare-up destined to burn out. It was the construction of a lasting infrastructure of defense inside the tumor and throughout the body.
Why This Matters
One of the greatest challenges in oncology is preventing relapse. Even after successful treatment, dormant cancer cells can linger, hidden, and then return with renewed aggression months or years later. A therapy that not only kills cancer cells but also leaves behind an immune memory could be transformative.
Masanobu Komatsu, Ph.D., principal investigator of the study and senior scientist at the Johns Hopkins All Children’s Cancer & Blood Disorders Institute, put it simply: “By building the right immune infrastructure inside tumors, we can potentiate the patient’s own defenses—both T cell and B cell arms—against cancer growth, relapse, and metastasis.”
This approach may also synergize with existing treatments. Checkpoint inhibitors, which release the brakes on T cells, have revolutionized cancer care but work best in immune hot tumors. By artificially heating up cold tumors with TLS-inducing therapy, doctors could dramatically expand the reach of these immunotherapies. Traditional chemotherapy, too, might become more effective when paired with a stronger immune presence.
A Broadly Applicable Strategy
One of the most exciting aspects of this research is its potential generalizability. TLS abundance is linked to better outcomes across many cancer types. If dual activation of STING and LTβR can reliably induce TLSs, it may become a universal tool to enhance therapy, not limited to one or two cancers. Breast, pancreatic, muscle, and possibly many other tumor types could all benefit from the same principle: turn cold into hot, and let the immune system do its work.
Looking Toward the Future
Of course, this is early research. While the mouse models show remarkable promise, translation into human patients will take time and rigorous testing. The team at Johns Hopkins is already investigating the mechanisms in more detail and preparing for clinical applications in both adult and pediatric cancer patients.
There are also practical questions: how best to deliver the stimulators, how to control for safety, and how to combine the therapy with other treatments. One of the co-authors of the study has disclosed potential competing interests, underscoring the importance of careful oversight as the work moves forward.
Yet the vision is clear: if scientists can reprogram the immune system inside tumors, they may unlock a new era of durable, personalized cancer therapy.
The Emotional Weight of Discovery
Cancer is not just a scientific problem—it is deeply human. Every patient, every family touched by the disease knows the fear of recurrence, the fragility of remission. The idea that the body could be taught to remember and resist cancer, much as it remembers viruses after vaccination, is both scientifically thrilling and emotionally profound. It suggests a future where survival is not temporary, where the immune system itself stands guard long after treatment ends.
For centuries, medicine has sought ways to outsmart cancer. Now, with the tools of immunology, we are beginning to see how the body itself can be empowered to win the fight. This study is not the end of that journey, but it is a vital step forward—a reminder that even the most immune-cold tumors can be taught to burn with new life, and that hope, like the immune system itself, can be reignited.
More information: Junko Sawada et al, Simultaneous STING and lymphotoxin-β receptor activation induces B cell responses in tertiary lymphoid structures to potentiate antitumor immunity, Nature Immunology (2025). DOI: 10.1038/s41590-025-02259-8