Some cancers are notoriously elusive, shifting form and evading treatments that would normally target them. This is especially true for carcinomas, a group of tumors that behave in bizarre, unpredictable ways. These tumors can almost seem to “change shape” — turning into cells that resemble those from other parts of the body. It’s a strategy that helps them slip under the radar of therapies designed to stop them.
But in the war against cancer, new hope is emerging from an unlikely place: the understanding of how these tumors manipulate their very identity.
Professor Christopher Vakoc from Cold Spring Harbor Laboratory (CSHL) has spent years studying the fundamental behaviors of these enigmatic cancer cells. “The tumors are notoriously plastic in their cellular identity,” he says, reflecting on their chameleon-like ability to morph and escape detection. In some cases, this transformation allows the tumors to outsmart conventional treatments, making them especially dangerous.
A Deeper Dive into Cellular Identity
Vakoc’s latest research, however, is unveiling cracks in the armor of these tumors, revealing unexpected vulnerabilities. In a pair of breakthrough studies, his team has uncovered key insights that could one day lead to therapies designed specifically to target these tricky, shapeshifting cancer cells.
In a paper published in Nature Communications, researchers from the Vakoc lab made a significant discovery involving pancreatic cancer, one of the most notoriously aggressive forms of cancer. The team identified a protein that plays a crucial role in determining whether pancreatic cancer cells maintain their usual form or whether they “switch” and adopt the characteristics of skin cells. This transformation may be one reason why pancreatic tumors are so difficult to treat. The ability of these cells to change their identity gives them an advantage when it comes to evading therapy.
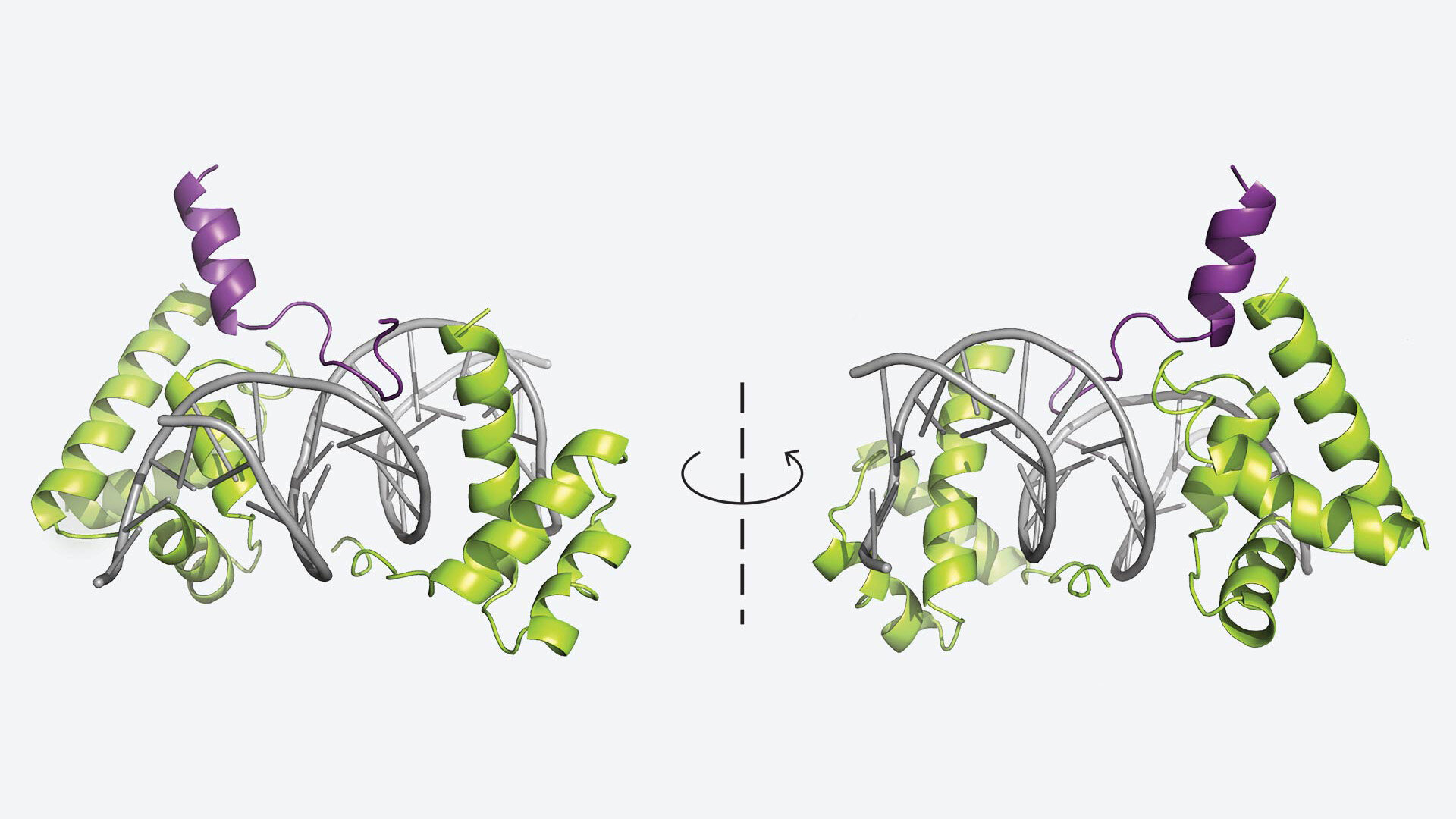
Meanwhile, in another study published in Cell Reports, Vakoc’s team uncovered the crystal structure of a protein that plays a vital role in tuft cell lung cancer — a rare and aggressive form of cancer. This discovery represents a full-circle moment for the lab. Back in 2018, Vakoc and his team had first identified tuft cell lung cancer while studying epigenetic factors that influence tumor growth. By examining the processes that regulate gene expression, they were looking for new avenues to understand and potentially control cancer.
Now, these findings have added a critical piece to the puzzle. Together, these studies shed new light on the fundamental mechanisms that allow cancers like pancreatic and lung tumors to evolve and become resistant to treatment.
Rewriting the Rules of Cancer Treatment
Vakoc and his collaborators are not only discovering new ways to understand cancer—they’re also laying the groundwork for more targeted therapies. In their pursuit to better understand how cancer cells change their identities, they’ve uncovered potential therapeutic targets that could one day lead to drugs designed to halt the progression of these aggressive cancers.
This focus on cellular identity is personal for Vakoc, who has spent over 17 years trying to identify the “master regulators” behind tumor behavior. These master regulators are the key drivers of cellular identity, the factors that determine whether a cell remains in its original form or morphs into something else. If scientists can identify and target these regulators, it could open the door to a new class of cancer treatments.
“We aim to identify the master regulators of cellular identity,” Vakoc says, referring to the central goal that has guided his work. This approach is not entirely new, but Vakoc’s lab is taking it a step further by looking beyond the genes themselves and focusing on the processes that control them—the transcription and gene regulation mechanisms that govern how a cell expresses its genetic material.
The hope is that these discoveries could lead to new therapies that act like hormone treatments used for breast and prostate cancer—therapies that target the cancer without harming surrounding healthy tissue. And while there’s still much work to be done before these therapies are ready for clinical use, the progress so far is promising.
High-Specificity Treatments: A New Standard
One of the key values driving Vakoc and his team is the desire to set a new, higher bar for cancer treatment. Unlike conventional therapies that often cause side effects due to their broad targeting, Vakoc’s team is focusing on specificity. They’re developing therapies that precisely target the cancer cells, sparing healthy tissues in the process.
In their most recent studies, whether they were working with mouse models of pancreatic cancer or lung cancer, they found no signs of toxicity or damage to vital organs. This is a game-changer in the world of cancer therapy, where many treatments come with harsh side effects.
“We’re setting a higher bar for specificity when it comes to new cancer targets and treatments,” Vakoc explains. This mindset could lead to a future where cancer patients receive more effective treatments with fewer harmful side effects—treatments that don’t just fight the cancer but do so with remarkable precision.
Why This Research Matters
For years, cancers like pancreatic and lung cancers have been some of the most difficult to treat. These tumors are known for their ability to evolve and adapt, making them highly resistant to conventional therapies. But the new insights coming from Vakoc’s lab offer a glimmer of hope for patients battling these aggressive cancers.
Understanding how cancer cells change their identity opens up new avenues for treatment. If researchers can target the “master regulators” of cellular identity, they may be able to stop tumors from morphing into more dangerous forms, giving doctors a powerful tool to combat cancer more effectively.
But this research does more than just offer new treatments—it challenges us to rethink the way we approach cancer itself. By studying the very mechanisms that allow tumors to escape treatment, Vakoc and his team are forging a new path in cancer research. Their work is laying the groundwork for a future where cancer is not just treated, but understood and managed with unprecedented precision.
In a field where progress often feels slow and hard-won, these new findings are a reminder that the battle against cancer is far from over—and that there are still many more breakthroughs to come.
More information: Patrick J. Cunniff et al, KLF5 enables dichotomous lineage programs in pancreatic cancer via the AAA+ ATPase coactivators RUVBL1 and RUVBL2, Nature Communications (2025). DOI: 10.1038/s41467-025-66007-0
Aktan Alpsoy et al, Structural basis of DNA-dependent coactivator recruitment by the tuft cell master regulator POU2F3, Cell Reports (2025). DOI: 10.1016/j.celrep.2025.116572