In the winding canyons of the human brain, glioblastoma multiforme (GBM) lurks like a cunning predator—stealthy, aggressive, and devastatingly elusive. It is the most common and deadliest form of malignant brain tumor in adults, with median survival measured in months, not years. For decades, medicine has hurled its most sophisticated weapons at GBM—surgery, radiation, chemotherapy, and even immunotherapy—but the cancer continues to outmaneuver them all.
Now, in a revelatory new study published in Nature, researchers have cracked open a startling biological secret: glioblastoma doesn’t fight alone. It enlists the brain’s own astrocytes—star-shaped support cells long considered the brain’s housekeepers—to quietly sabotage the immune system from within. By recruiting these astrocytes, GBM creates a microenvironment that silences immune attack and clears the way for its rapid, deadly expansion.
This discovery doesn’t just rewrite how we understand GBM—it opens a door to a radically new therapeutic frontier, one that could finally turn the brain’s own architecture against the tumor that hides within it.
The Puzzle of the Immune-Proof Tumor
For years, the puzzle baffled oncologists: why is GBM so resistant to immunotherapies that have revolutionized treatment for cancers like melanoma and lung cancer? Immune checkpoint inhibitors, CAR-T cell therapies, and cancer vaccines have proven astonishingly effective elsewhere—but in the brain, their impact is minimal. The answer, as the new study reveals, may lie not in the immune system’s failure, but in its active sabotage by the very brain cells meant to support and protect neurons.
Astrocytes are the most abundant glial cells in the brain. Traditionally viewed as the brain’s caretakers—maintaining the blood-brain barrier, supporting neurons, and responding to injury—astrocytes are now emerging as active regulators of the immune system. In diseases like multiple sclerosis and Alzheimer’s, astrocytes are known to influence inflammatory responses. But in cancer, their role has remained enigmatic.
That is, until now.
Decoding the Astrocyte Conspiracy
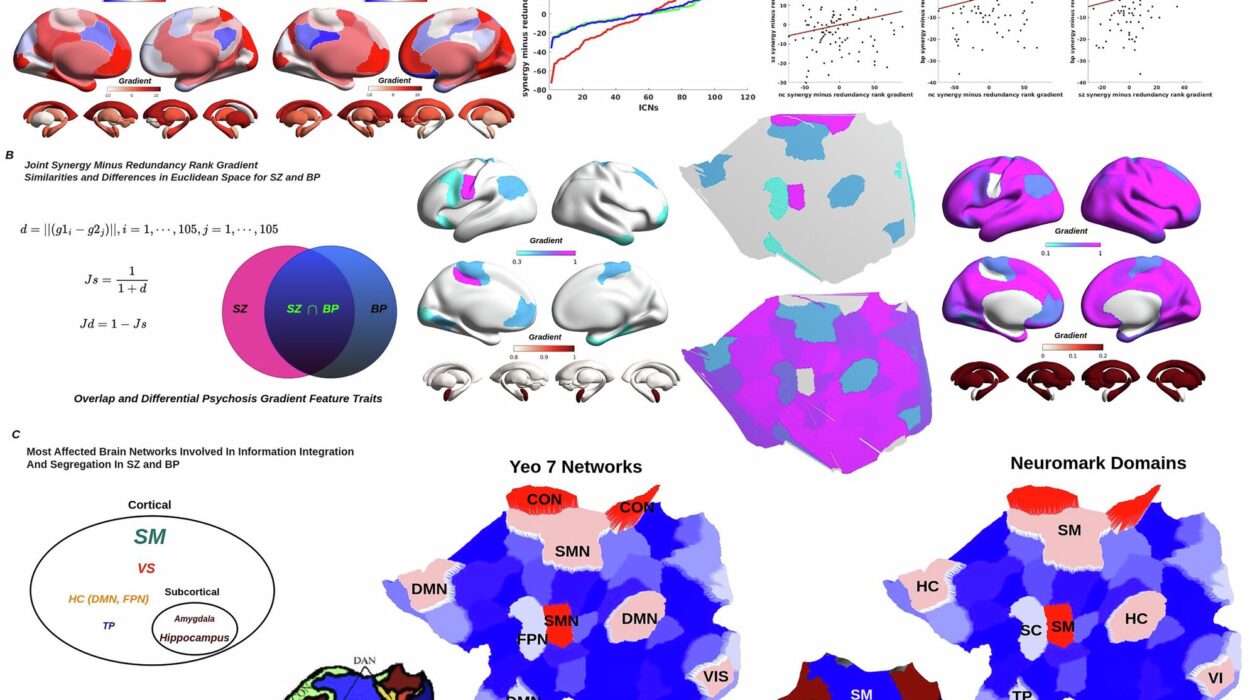
Led by a team of neuroscientists, oncologists, and immunologists, the researchers used a multidimensional arsenal of technologies to peer deep into the tumor microenvironment. Using high-resolution single-cell RNA sequencing, multiplexed imaging, and advanced in vivo models, they profiled astrocytes in both human GBM tissue samples and mouse models designed to mimic the disease.
What they found was nothing short of astonishing: a distinct subset of astrocytes had taken on a dark role. These weren’t the supportive, neuroprotective cells described in textbooks. These astrocytes had been hijacked. Transformed by signals from the tumor, they acquired the ability to actively suppress T cells—the immune system’s front-line soldiers.
This discovery turned existing paradigms upside down. Rather than acting as neutral bystanders or helpful responders, these astrocytes had become agents of immune suppression, complicit in the cancer’s survival.
The Role of IL-11: Glioblastoma’s Secret Weapon
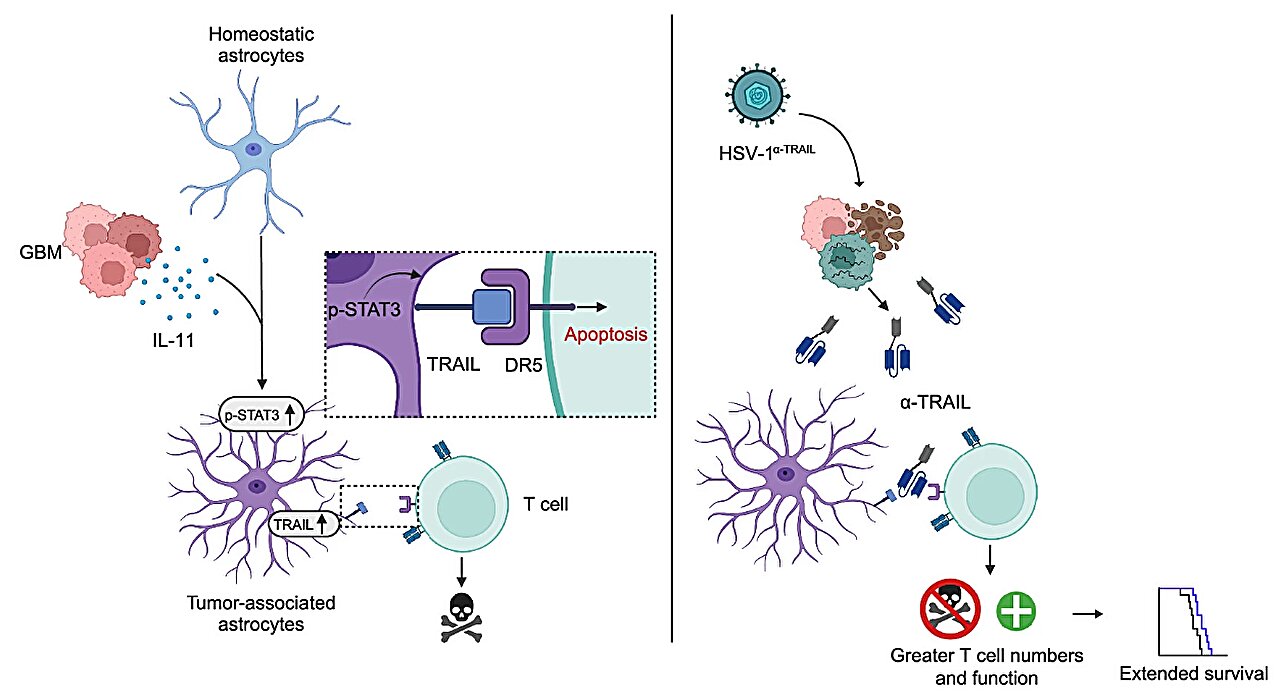
Digging deeper, the team identified the molecular spark that triggered this transformation: interleukin-11 (IL-11), a potent signaling molecule secreted by the tumor itself. IL-11, previously associated with inflammatory and fibrotic processes, was being pumped out by GBM cells into the surrounding brain tissue. The effect was chillingly precise—IL-11 activated the T cell–killing astrocytes, effectively flipping their switch from helper to saboteur.
The presence of IL-11 correlated with worse clinical outcomes: shorter survival times, faster tumor recurrence, and more aggressive disease progression. It was as though GBM had evolved a molecular whisper, capable of turning the brain’s support network into its own private army.
Genetic Deletion and Viral Counterattack
To test whether these immunosuppressive astrocytes were truly critical to the tumor’s survival, the researchers used genetic tools to selectively eliminate them in mouse models. The results were dramatic. With the T cell–killing astrocytes deactivated, immune cells flooded into the tumor environment. T cells survived longer, multiplied more aggressively, and attacked the tumor with renewed vigor. The tumors shrank, survival increased, and recurrence was delayed.
This wasn’t just a biological insight—it was a therapeutic opportunity.
To take the concept even further, the team designed an innovative viral therapy. Using oncolytic viruses—genetically engineered viruses that selectively infect and kill cancer cells—they programmed the viruses to produce an antibody that blocks IL-11 signaling right inside the tumor. This meant the virus could both attack tumor cells directly and disarm their astrocytic protectors at the same time.
The results were striking. Treated mice showed robust immune activation, dramatically improved survival, and, in some cases, long-term remission. It was a proof-of-concept that astrocyte-targeted immunotherapy could reshape the battlefield of GBM treatment.
Rethinking the Brain’s Immune Landscape
This study marks a turning point in neuro-oncology. For decades, the brain was thought to be an “immune-privileged” organ—sealed off from immune surveillance, protected by the blood-brain barrier. But in reality, the brain’s immune system is dynamic, interconnected, and, as this research shows, vulnerable to manipulation.
The glioblastoma tumor microenvironment is not just a passive setting—it’s an active participant in immune evasion. Astrocytes, once believed to be defenders of the central nervous system, are now known to be double agents under the tumor’s control.
Moreover, the role of IL-11 is likely just the beginning. The study’s authors are now investigating how IL-11 affects other cells in the tumor microenvironment, including microglia, endothelial cells, and infiltrating immune cells. They also plan to explore IL-11’s role in brain metastases—cancers that spread to the brain from other organs—and whether this astrocyte hijacking mechanism is a broader strategy used across multiple cancer types.
Toward Astrocyte-Targeted Immunotherapy
The implications of this work are profound. For the first time, researchers have a mechanistic roadmap of how GBM turns the immune system against itself. By targeting IL-11 and the T cell–killing astrocyte subset, new immunotherapies could be developed that don’t just aim at the cancer cells but at the cancer’s immune camouflage.
Such therapies could be used alongside existing treatments—like checkpoint inhibitors or radiation therapy—to strip away GBM’s protective shell and expose it to the full force of the immune system. They could also be adapted to other brain tumors and possibly non-neurological cancers with similar microenvironmental strategies.
This astrocyte-targeted approach is more than a scientific breakthrough—it is a paradigm shift. It forces researchers to look beyond cancer cells themselves and toward the ecosystem they manipulate. The future of brain cancer treatment may not lie solely in attacking tumors head-on, but in disrupting their invisible alliances.
Conclusion: A New Frontier in the War on Glioblastoma
Glioblastoma has long stood as a fortress at the edge of medicine’s reach. But this new research tears down part of that wall, exposing the subtle, cellular conspiracies that shield the tumor from harm. By shining light on the shadowy role of astrocytes and decoding the language of IL-11, scientists have uncovered a hidden layer of complexity—and a powerful new weakness to exploit.
It’s a discovery born of persistence, precision, and an unflinching willingness to look where few dared to look: not just at the cancer, but at the brain itself. The fight against glioblastoma has always been daunting, but with the brain’s own machinery finally turned against the enemy, a new kind of hope emerges—not just for prolonging life, but for rewriting the rules of cancer resistance altogether.
Reference: Camilo Faust Akl et al, Glioblastoma-instructed astrocytes suppress tumour-specific T cell immunity, Nature (2025). DOI: 10.1038/s41586-025-08997-x