Cancer diagnosis has always relied heavily on the trained eyes of pathologists, who examine stained tissue sections under a microscope, searching for subtle changes that reveal the disease’s secrets. In colorectal cancer—the third most common cancer worldwide—these slides contain not only visible signs of disease but also hidden genetic clues that determine how the cancer behaves and how it should be treated.
Now, an international team of researchers has taken a major step forward by teaching artificial intelligence (AI) to read these clues directly from routine pathology slides. Their work, published in The Lancet Digital Health, could pave the way for faster, more cost-effective, and more personalized cancer care.
The Study That Changed the Landscape
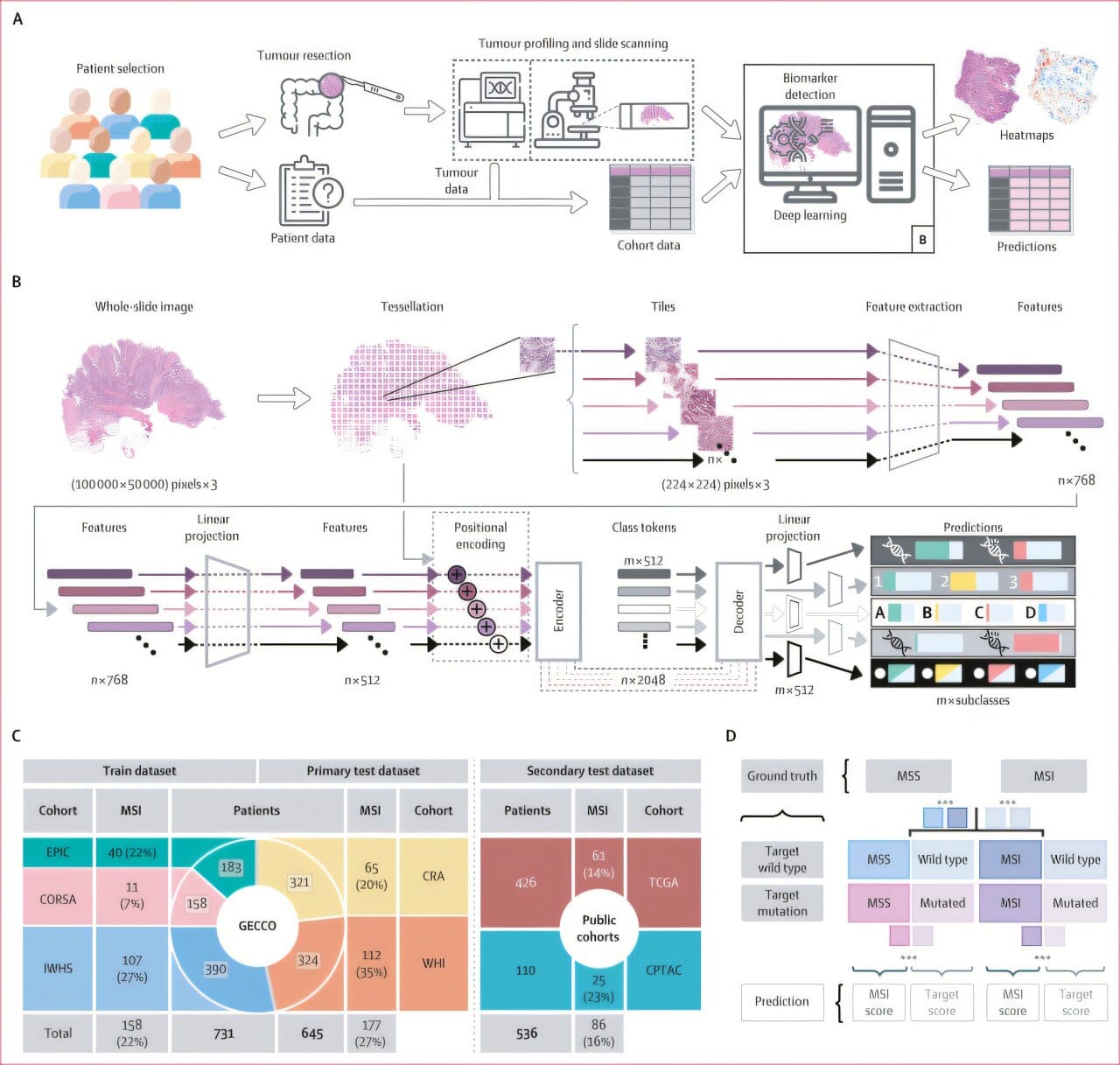
In one of the largest studies of its kind, scientists analyzed nearly 2,000 digitized tissue slides from colon cancer patients across seven independent cohorts in Europe and the United States. These were not just images of stained tissues; the researchers also integrated clinical, demographic, and lifestyle information, creating a rich dataset that reflects the complexity of real-world patients.
The key innovation was the development of a novel “multi-target transformer model”—a type of deep learning AI that can predict many genetic alterations simultaneously, rather than focusing on just one. Previous models could only identify individual mutations, ignoring the fact that cancers often carry multiple co-occurring mutations that collectively shape tumor behavior.
By capturing shared morphological patterns in tissue—the subtle ways that cells arrange, deform, and interact—the model was able to detect not just known genetic alterations, but also emerging biomarkers that may become clinically important in the future.
Why Genetic Alterations Matter
Every cancer is driven by genetic mutations that cause cells to grow uncontrollably. In colorectal cancer, certain mutations influence how aggressive the disease becomes, how it spreads, and how it responds to treatment. For example, patients with BRAF mutations often face worse outcomes, while those with microsatellite instability (MSI) may respond exceptionally well to immunotherapy.
Microsatellites are short, repetitive stretches of DNA scattered throughout the genome. Normally, cells have a repair system that keeps these sequences stable. But when that system fails, MSI arises—leaving behind a genetic fingerprint that can be detected in tumor tissue. MSI status is now a crucial biomarker in treatment planning, helping oncologists decide whether a patient is likely to benefit from immune checkpoint inhibitors.
Traditionally, identifying such biomarkers requires expensive and time-consuming molecular tests. The new AI model, however, can infer them directly from routine pathology slides, potentially reducing costs and making advanced diagnostics more accessible worldwide.
The Human Effort Behind the AI
Although the study showcases cutting-edge technology, its success also depended on deep human expertise. Pathologists played a central role, ensuring that the AI learned from accurately labeled images and guiding the interpretation of its predictions. Dr. Nic Reitsam from the University Hospital Augsburg was instrumental in providing this pathological expertise, bridging the gap between clinical practice and computational science.
The project also exemplified teamwork across disciplines. Data scientists, epidemiologists, oncologists, and digital health experts collaborated closely, combining their knowledge to create a model that is scientifically robust and clinically meaningful.
Exceeding Expectations
The multi-target transformer model was rigorously validated across multiple patient cohorts. The results were striking: not only did the model match established single-target AI systems in predicting biomarkers like BRAF and RNF43 mutations, but in some cases it even outperformed them.
Equally important, the model captured the collective influence of multiple mutations on tissue morphology. As first author Marco Gustav, M.Sc., from TU Dresden explained, “Earlier models looked at one mutation at a time. Our approach shows that mutations interact and leave behind shared visual patterns, which AI can detect more effectively than humans.”
This holistic perspective represents a shift in cancer diagnostics, moving away from one-to-one associations and toward an integrated view of the tumor as a dynamic system.
Implications for Patients
For patients, the promise of this technology is profound. Faster and more affordable testing means earlier identification of key biomarkers, which can guide personalized treatment decisions. Instead of waiting weeks for genetic test results, a pathologist may soon have an AI-assisted tool that delivers insights within hours of examining tissue slides.
Professor Jakob N. Kather, senior oncologist at TU Dresden and leader in clinical AI research, emphasized the transformative potential: “Our research shows that AI models can significantly accelerate diagnostic workflows. At the same time, these methods provide new insights into how genetic changes shape the visible architecture of tumors.”
Such advances could democratize precision medicine, making state-of-the-art care available not only in well-funded hospitals but also in resource-limited settings where genetic testing is often unavailable.
Looking to the Future
While this breakthrough focuses on colorectal cancer, the team has set its sights on broader horizons. The same approach could be extended to other cancers, where histological slides are already a cornerstone of diagnosis. Breast, lung, and prostate cancers are just a few examples where AI-based multi-target prediction could help decode complex genetic landscapes.
There is still work to be done before such tools become standard in clinical practice. Regulatory approval, clinical trials, and careful integration into medical workflows will be required. Yet the progress made in this study signals a future where AI does not replace pathologists but becomes their trusted partner—augmenting human expertise with computational power.
A Bridge Between Science and Care
The story of this study is more than a tale of algorithms and datasets. It is a reminder of how human ingenuity, compassion, and collaboration can harness technology for the benefit of patients. By blending the wisdom of medicine with the innovation of AI, researchers have opened a new chapter in the fight against cancer.
The microscope has been the pathologist’s window into disease for over a century. With the advent of AI, that window is now wider and clearer than ever before. The hidden genetic stories of cancer are becoming visible—not just to the trained human eye, but to intelligent systems that can learn, adapt, and reveal insights that once seemed beyond reach.
In the end, the true power of this discovery lies not only in advancing science but also in offering hope: hope for quicker diagnoses, more tailored treatments, and better outcomes for patients and families around the world.
More information: Marco Gustav et al, Assessing genotype−phenotype correlations in colorectal cancer with deep learning: a multicentre cohort study, The Lancet Digital Health (2025). DOI: 10.1016/j.landig.2025.100891