Every stage of motherhood—pregnancy, breastfeeding, and the transition afterward—is a profound transformation, not just in a woman’s life but deep within her biology. Among the many changes that unfold, the mammary gland undergoes one of the most dramatic evolutions in the human body. This organ, so central to nurturing new life, reshapes itself completely depending on whether it is preparing for milk production, actively nourishing an infant, or returning to rest.
Yet until now, the intricate genetic choreography behind this process remained largely hidden. A new study in mice, published in Nucleic Acids Research, has shed light on the genes that orchestrate this transformation. By mapping the mammary gland across its entire adult developmental cycle, scientists have created the most detailed genetic atlas of its kind—one that could reshape how we understand breastfeeding, maternal health, and even breast cancer.
Mapping a Cycle of Transformation
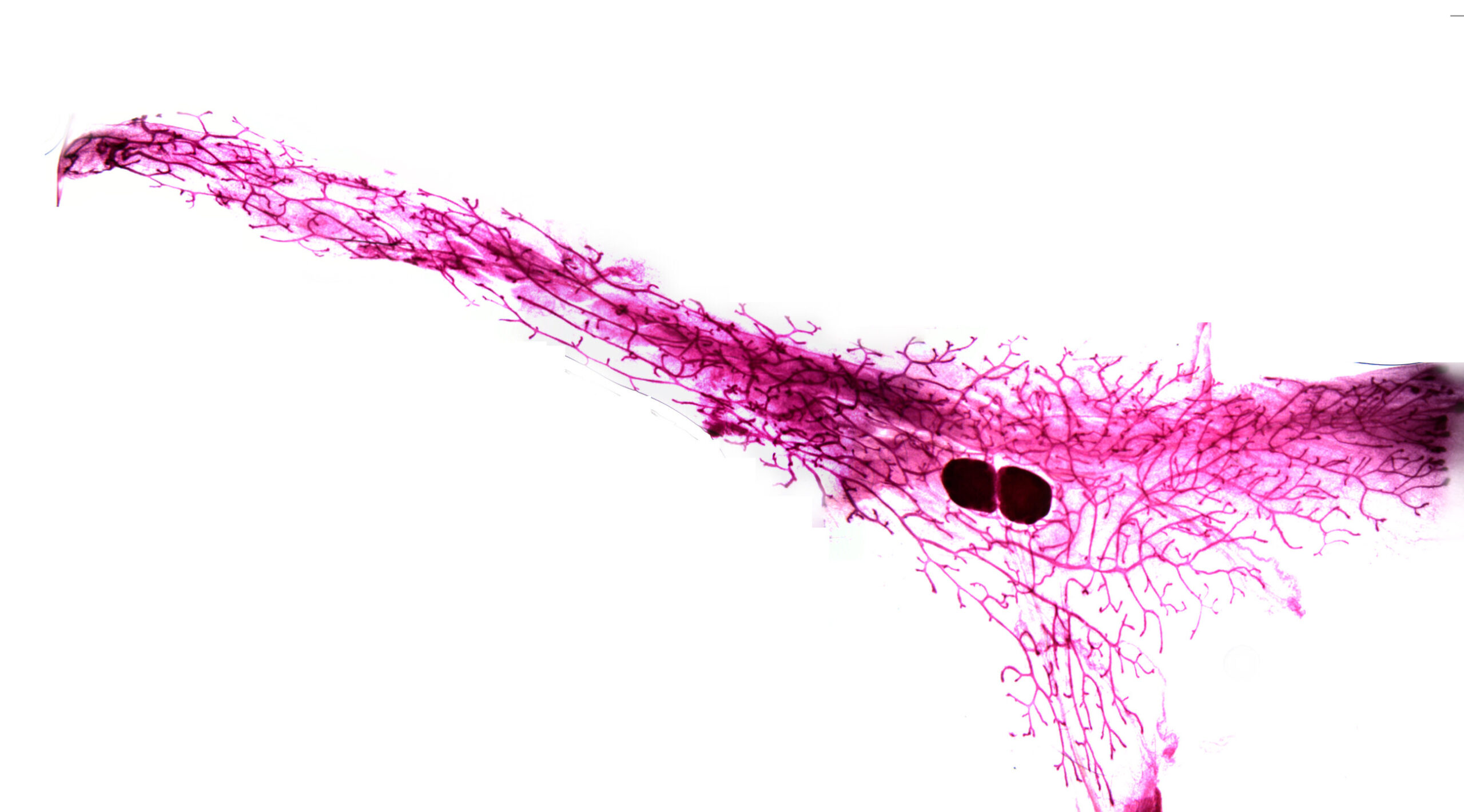
The mammary gland is far more than a milk-producing organ. It is a complex community of different cell types working in harmony. Fat cells provide the structure that holds everything together. Specialized basal cells contract rhythmically to push milk outward during feeding. Secretory cells manufacture milk itself, packing it with the nutrients an infant needs. Each type of cell plays a role in a cycle that begins before pregnancy, accelerates during gestation, peaks in breastfeeding, and winds down through a process called involution, when the breast remodels itself to its resting state.
In this new study, researchers examined the mammary gland at ten distinct time points throughout this cycle, carefully analyzing both the types of cells present and the genes active within them. By comparing these snapshots, they could trace how genes “switch on” and “switch off” as the organ changes roles—shifting from preparation, to nourishment, to recovery.
As lead author Dr. Geula Hanin of the University of Cambridge explained, the result is a map that allows scientists to pinpoint exactly which genes are expressed in which cells at each stage of the journey. It is, in effect, a genetic time-lapse of motherhood at the cellular level.
Breastfeeding Difficulties: A New Perspective
One of the most striking discoveries relates to breastfeeding challenges. Up to one in twenty women experience difficulty in producing or delivering enough milk, a problem that can cause distress for both mother and baby. Traditionally, such issues have been blamed on the milk-producing cells themselves. But the new genetic atlas tells a more complex story.
The researchers found that genes linked to breastfeeding problems were also active in other cell types, including basal cells—the ones responsible for physically ejecting milk as the baby suckles. This suggests that in some cases, breastfeeding struggles may not be due to insufficient milk production, but rather to a mechanical difficulty in releasing it.
This subtle shift in perspective could open new avenues for treatment and support. Instead of focusing only on boosting milk supply, future interventions might also target the mechanics of milk release, offering mothers more comprehensive solutions.
The Shadow of Postpartum Breast Cancer
The atlas also uncovered insights into one of the most devastating health risks for new mothers: postpartum breast cancer. This form of cancer, which can appear within five to ten years of childbirth, has long been linked to the dramatic hormonal shifts and tissue remodeling that occur during involution.
What the new study revealed is that genes associated with postpartum breast cancer switch on immediately after weaning, and not just in the milk-producing cells. Surprisingly, fat cells—often considered passive bystanders—also showed cancer-linked genetic activity. This finding challenges previous assumptions and highlights fat cells as potential players in the disease.
By identifying these overlooked contributors, the atlas provides new targets for early detection and prevention strategies. In the future, monitoring genetic activity in these cells could help doctors predict which women are at higher risk and intervene before cancer develops.
The Role of Imprinted Genes
Another intriguing discovery centers on “imprinted genes.” These are genes that behave differently depending on whether they are inherited from the mother or the father. In the placenta, imprinted genes are already known to regulate fetal growth and development. Now, the study has shown that 25 such genes play crucial roles in the mammary gland during adulthood.
These imprinted genes switch on at precise moments in the cycle, guiding processes like milk production and tissue remodeling. They act almost like conductors of an orchestra, ensuring that the different players—the various cell types—perform in harmony at the right time. This insight deepens our understanding of how tightly regulated the mammary gland is, and how delicate imbalances could lead to health problems.
Why It Matters for Mothers and Infants
Breastfeeding is more than nourishment; it is a foundation for lifelong health. Babies who are breastfed are less likely to develop obesity and diabetes later in life. For mothers, breastfeeding is linked to reduced risks of certain cancers and metabolic diseases. Despite these benefits, breastfeeding remains a challenge for many women, and scientific research into the underlying biology has historically been underfunded and overlooked.
This new genetic atlas is a step toward changing that. By uncovering how genes shape the mammary gland’s cycle, scientists now have a roadmap for understanding both normal function and dysfunction. It could help predict breastfeeding problems before they occur, guide new therapies, and reduce the stigma mothers often feel when they face difficulties. It could also illuminate new strategies for preventing postpartum breast cancer—a disease that strikes at a vulnerable time in a woman’s life.
A Glimpse Into the Future
The mammary gland, often studied primarily for its role in lactation, is emerging as a model for how organs adapt, transform, and heal. This atlas shows that its complexity rivals that of other major organ systems, and that its secrets have far-reaching implications for health.
As Dr. Hanin, who co-leads the Cambridge Lactation Network, emphasized, breastfeeding is a universal process across mammals. Humanity’s very survival has depended on it for millennia. By unlocking the genetic blueprint behind it, researchers are not just solving a scientific puzzle—they are supporting the fundamental act of nurturing life.
The atlas is not the end of the story but the beginning. Each gene identified, each cell mapped, is a thread in a larger tapestry. In the years to come, this knowledge may transform maternal and infant health, bringing science and compassion together to support mothers in one of the most extraordinary journeys the body can take.
More information: Geula Hanin et al, Dynamic Allelic Expression in Mouse Mammary Gland Across the Adult Developmental Cycle, Nucleic Acids Research (2025). DOI: 10.1093/nar/gkaf804