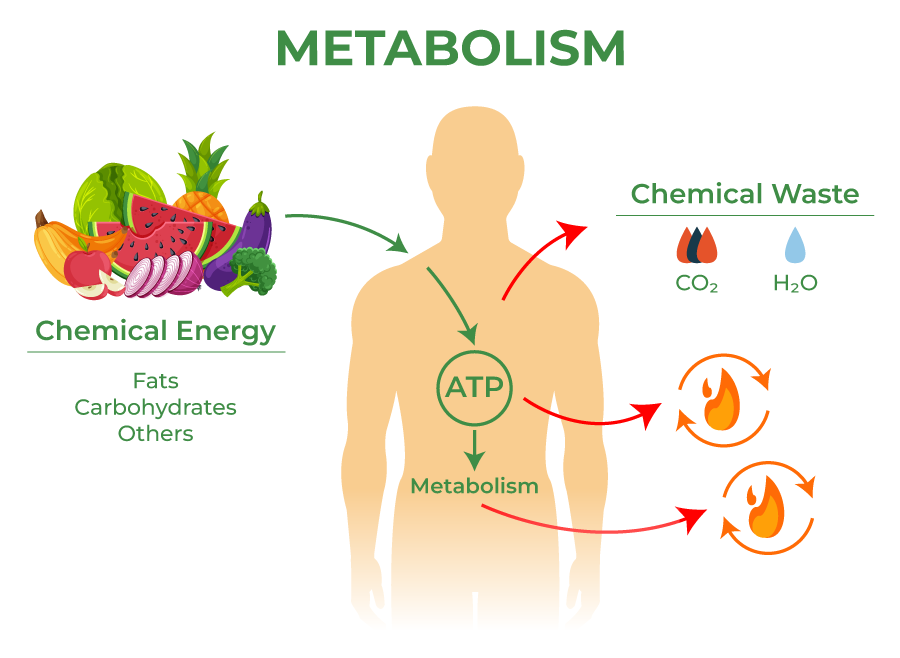
Inside every living cell, a continuous dance of molecules fuels the body’s life-sustaining operations. This constant hum of chemical activity, known as metabolism, transforms the food we eat into energy and building blocks, and breaks down waste to maintain cellular function. Metabolism is life in motion—without it, even the most basic biological processes would cease. But increasingly, scientists are uncovering that metabolism may do more than just keep us alive. It might also dictate how quickly we age and how soon our bodies begin to falter.
Aging has long been considered one of biology’s greatest mysteries. Once thought to be a passive accumulation of wear and tear, aging is now seen as a regulated process shaped by genetics, environment, and, crucially, metabolism. In this new paradigm, the rate at which we burn energy, manage nutrients, and handle metabolic stress could be the very key to extending life and delaying age-related decline.
The Curious Case of the Calorie
One of the first and most enduring clues linking metabolism to aging came from the unlikely field of dietary restriction. In the 1930s, researchers discovered that mice fed fewer calories lived significantly longer than their well-fed counterparts. Over the decades, calorie restriction has been shown to extend lifespan in yeast, worms, flies, and rodents. In primates, the results are mixed but promising.
What makes this so fascinating is not just the idea that “eating less makes you live longer,” but the implication that nutrient intake directly alters the body’s metabolic machinery in ways that affect aging. When the body is forced to operate on fewer calories, it slows down certain metabolic pathways, increases efficiency, and ramps up cellular repair mechanisms. It’s as if the body, sensing a time of scarcity, becomes more cautious, more conservative, and more invested in self-preservation.
This shift is not just about energy. It’s about signaling. Nutrients trigger hormones and molecular pathways that send messages across cells and tissues. When those signals are adjusted—whether by diet, genetics, or pharmaceutical intervention—they can tilt the balance between aging and longevity.
Mitochondria as the Power Plants of Longevity
Deep inside cells lie mitochondria, often described as the powerhouses of the cell. These organelles generate most of the cell’s energy through a process called oxidative phosphorylation, which converts nutrients into ATP—the molecule that powers almost every biological reaction.
But mitochondria are more than just energy generators. They are also central players in the aging process. One reason is that as they produce energy, mitochondria generate reactive oxygen species (ROS), unstable molecules that can damage DNA, proteins, and lipids. For a long time, these molecules were thought to be villains in the story of aging, sparking the so-called “free radical theory” that oxidative stress drives cellular aging.
But this theory has evolved. While high levels of ROS are clearly harmful, moderate levels may actually trigger protective responses—a phenomenon called mitohormesis. When cells experience mild stress, they activate antioxidant defenses, repair mechanisms, and anti-aging pathways. In this light, mitochondria become double agents: dangerous when damaged or overworked, but beneficial when gently challenged.
Mitochondrial dysfunction, on the other hand, is a hallmark of aging. As we grow older, our mitochondria lose efficiency, produce more ROS, and accumulate mutations in their DNA. This sets off a cascade of metabolic changes that ripple through tissues, leading to fatigue, cognitive decline, muscle wasting, and chronic disease. Preserving mitochondrial health is increasingly seen as a cornerstone of slowing the aging process.
Nutrient Sensing Pathways and the Biological Clock
In the last two decades, researchers have identified several key nutrient-sensing pathways that act as metabolic timekeepers. These include the insulin/IGF-1 pathway, mTOR (mechanistic Target of Rapamycin), AMPK (AMP-activated protein kinase), and sirtuins. Each of these systems responds to changes in nutrient availability and energy status and plays a role in regulating growth, repair, and aging.
The insulin/IGF-1 pathway is involved in glucose metabolism and cellular growth. When nutrients are abundant, this pathway is active, promoting cell division and anabolic processes. But when it’s dialed down—such as during fasting or low-calorie diets—cells shift toward maintenance and stress resistance. In multiple organisms, reduced IGF-1 signaling is linked to longer lifespans.
mTOR is another major player. It integrates signals from amino acids, energy levels, and growth factors to control protein synthesis and autophagy—the process by which cells recycle their damaged components. Inhibition of mTOR, whether through genetic manipulation or drugs like rapamycin, extends lifespan in numerous species. It’s a case of slowing down the machinery of growth to prioritize self-renewal.
AMPK acts like a cellular fuel gauge. When energy is low, AMPK is activated, promoting energy production and reducing energy-consuming processes. It boosts mitochondrial biogenesis, stimulates fat burning, and supports autophagy—all of which are associated with healthy aging.
Sirtuins are a family of enzymes that depend on NAD+, a molecule whose levels decline with age. Sirtuins influence DNA repair, mitochondrial function, inflammation, and metabolism. Activating them—whether through fasting, exercise, or molecules like resveratrol—has been shown to improve healthspan and potentially extend lifespan in animal models.
Together, these nutrient-sensing pathways form a metabolic network that not only governs how cells use energy, but also when and how they age.
The Slow Burn of Metabolic Rate
A long-standing theory in aging research is that a faster metabolism leads to a shorter lifespan, an idea supported by observations in animals. Mice, for example, have incredibly fast metabolic rates and live just a few years. Elephants and whales, with much slower metabolisms, live for decades. Birds are a curious exception—they have high metabolic rates but can live surprisingly long lives, suggesting the relationship is more nuanced.
Some of the most compelling evidence for this theory comes from studies of metabolic rate and lifespan in different species. In general, smaller animals with high energy turnover tend to burn out faster. Their cells generate more ROS, accumulate damage quicker, and undergo more rapid turnover. Larger animals, or those with slower basal metabolic rates, seem to age more slowly.
But this doesn’t mean humans should try to suppress their metabolism. It’s not the total amount of energy burned, but how that energy is used. Efficiency, flexibility, and the ability to adapt to metabolic stress may matter more than raw speed. In this context, metabolic resilience—how well the body handles shifts in energy demand—could be a better predictor of healthy aging than metabolism alone.
Inflammation as the Metabolic Fallout of Aging
As metabolism slows and regulatory pathways falter with age, the body becomes increasingly prone to a simmering, chronic inflammation dubbed “inflammaging.” This low-grade inflammatory state arises from multiple sources—accumulated cell debris, misfolded proteins, dysfunctional mitochondria, and senescent cells that refuse to die but continue to secrete inflammatory signals.
These inflammatory messengers disrupt metabolic processes, interfere with insulin signaling, and accelerate tissue degeneration. The result is a metabolic-immune feedback loop: poor metabolic function fuels inflammation, which further impairs metabolism.
This loop underlies many age-related diseases, including type 2 diabetes, cardiovascular disease, Alzheimer’s, and cancer. It’s no coincidence that these conditions all have roots in metabolic dysregulation. Aging, then, may not just be a timeline of birthdays but a metabolic descent into inflammatory chaos.
Interventions That Rewrite the Metabolic Script
Understanding how metabolism shapes aging opens the door to interventions aimed at extending healthspan—the period of life free from disease. Caloric restriction remains the gold standard, but few people can sustain it long-term. Intermittent fasting and time-restricted feeding offer similar benefits by creating periods of metabolic rest.
Pharmacological approaches are also emerging. Metformin, a drug used to treat type 2 diabetes, activates AMPK and improves insulin sensitivity. Some studies suggest it may reduce cancer risk and extend lifespan. Rapamycin, an mTOR inhibitor, has shown dramatic life-extension effects in animals and is being tested in humans.
NAD+ precursors like NMN and NR aim to boost sirtuin activity and mitochondrial function. Although early results are promising, more research is needed to verify their impact on aging in humans.
Exercise, perhaps unsurprisingly, is one of the most powerful metabolic tools available. It activates AMPK, stimulates mitochondrial renewal, reduces inflammation, and enhances insulin sensitivity. Regular physical activity reshapes the metabolic landscape in ways that mimic many anti-aging interventions—without the need for pills or extreme diets.
The Microbiome as a Metabolic Mediator
Another emerging player in the metabolism-aging link is the gut microbiome. The trillions of microbes living in our intestines influence digestion, immune function, and nutrient absorption. As we age, our microbiome composition shifts, often becoming less diverse and more inflammatory.
These microbial changes affect metabolic health, contributing to insulin resistance, weight gain, and even neurodegeneration. Some studies have shown that transferring the microbiome from young to old mice can rejuvenate metabolic function. Diet, probiotics, and prebiotics may help restore microbial balance and improve metabolic resilience in aging individuals.
The idea that the microbes we harbor could influence our longevity is both humbling and empowering. It suggests that aging is not solely a human story but a collective one, shaped by the ecosystems within us.
Gender Differences in Metabolic Aging
Men and women do not age the same way, metabolically speaking. Hormones like estrogen and testosterone play critical roles in regulating metabolism, fat distribution, and muscle mass. As these hormones decline with age, men and women experience different patterns of metabolic dysfunction.
Women, for example, face increased risk of metabolic syndrome after menopause, partly due to the loss of estrogen’s protective effects on glucose and lipid metabolism. Men, on the other hand, may experience more gradual changes but often have earlier onset of cardiovascular issues.
Understanding these differences is crucial for developing personalized interventions. A one-size-fits-all approach to metabolic health and aging is likely to miss the nuances that make each body unique.
The Future of Metabolic Longevity
Science is still unraveling the intricate links between metabolism and aging, but a vision of the future is beginning to take shape. It’s a future where we can measure biological age more precisely than chronological age, using metabolic markers and molecular signatures. Where personalized metabolic profiles guide interventions tailored to each person’s needs.
Gene editing tools like CRISPR may one day correct metabolic mutations. Stem cell therapies could rejuvenate aging tissues. Artificial intelligence might help decode complex metabolic networks to find new targets for therapy.
But the most powerful tools may still be the oldest ones: eating mindfully, moving regularly, resting deeply, and cultivating habits that support metabolic harmony.
A Symphony of Fire and Time
In the grand orchestra of life, metabolism is the conductor—the unseen force coordinating every note of growth, repair, and decay. Aging is not a solo performance by genes or fate but a symphony played through our metabolic instruments. How we fuel our bodies, how we adapt to stress, how we balance energy and entropy—these choices echo through time, sculpting the arc of our health and the length of our lives.
To understand the link between metabolism and aging is to glimpse a more dynamic view of time itself—not as an unstoppable march, but as a dance we can influence, a rhythm we can retune.
In the fire of metabolism burns not just the energy of today, but the potential for a longer, healthier tomorrow.