From the earliest days of science, humans have been fascinated by extremes. We ask how fast something can go, how massive an object can become before it collapses, how small matter can be divided, and how cold anything can possibly get. Among these questions, few are as haunting and as deceptively simple as this one: why can’t we reach absolute zero temperature?
Absolute zero is not just another number on a thermometer. It is the deepest imaginable cold, a boundary written into the fabric of nature itself. It represents a state where all classical motion ceases, where thermal energy reaches its minimum possible value. Scientists can approach it, circle around it, and glimpse its strange effects, but they can never truly arrive. Absolute zero stands like a horizon in physics, forever visible, forever unreachable.
Understanding why absolute zero cannot be reached takes us on a journey through thermodynamics, quantum mechanics, and the philosophical meaning of limits in nature. It is a story of how temperature truly works, what motion really means, and how the universe quietly enforces its most fundamental rules.
What Temperature Really Is Beneath Our Intuition
To understand why absolute zero is unreachable, we must first strip away our everyday intuition about temperature. In daily life, temperature feels simple. Hot objects burn, cold objects chill, and thermometers give us numbers that seem straightforward. But temperature is not a substance or a vague sensation. It is a precise physical concept tied to motion and energy.
At its core, temperature measures the average kinetic energy of particles in a system. In solids, atoms vibrate about fixed positions. In liquids, they slide past one another. In gases, they rush freely through space. The faster these particles move on average, the higher the temperature. The slower they move, the colder the system becomes.
Cooling something means removing energy from it. When you put a hot object into a cold environment, energy flows out. The atoms vibrate less. Their motion becomes gentler, quieter, more restrained. But motion itself is not something that can simply be switched off. It is deeply woven into the nature of matter.
Absolute zero, defined as zero kelvin, corresponds to the theoretical state in which particles possess the minimum possible energy allowed by nature. It is not merely very cold. It is the point where classical thermal motion would disappear entirely. That is why it is such a powerful concept and such a profound challenge.
The Meaning of Absolute Zero in Physics
Absolute zero is defined as zero on the Kelvin temperature scale. Unlike Celsius or Fahrenheit, the Kelvin scale does not rely on arbitrary reference points like the freezing of water. Instead, it is anchored in fundamental physics. Zero kelvin is the lowest possible temperature, the point where thermal energy is minimized.
At absolute zero, entropy reaches its minimum value, and a system would be in its lowest energy state. From a classical perspective, atoms would be perfectly still, frozen in place with no vibration at all. This picture is simple and seductive, but it turns out to be incomplete.
The deeper meaning of absolute zero emerges when we look at the laws of thermodynamics. The third law of thermodynamics states that it is impossible to reach absolute zero through any finite number of physical processes. No matter how advanced our technology becomes, no matter how clever our cooling methods are, absolute zero remains out of reach.
This is not a limitation of engineering. It is a law of nature.
The Third Law of Thermodynamics and Nature’s Quiet Prohibition
The third law of thermodynamics is one of the most subtle and powerful statements in physics. It does not shout its authority. It simply states a quiet impossibility. As a system approaches absolute zero, the amount of work required to remove additional heat grows without bound. Each step closer becomes exponentially harder than the last.
Cooling works by transferring energy from a colder object to a warmer one. But as a system becomes extremely cold, there are fewer and fewer available states into which energy can be removed. The system resists further cooling, not actively, but structurally. Nature runs out of accessible pathways for energy to escape.
In practical terms, this means that no finite sequence of operations can ever remove the last remaining thermal energy. You can get incredibly close, unimaginably close, but never fully arrive. Absolute zero is not a destination. It is an asymptote.
This law reflects something profound about the structure of reality. It tells us that there are absolute limits, boundaries that cannot be crossed no matter how much effort we apply. Absolute zero is one such boundary.
Why Cooling Gets Harder the Colder You Go
To understand why cooling becomes increasingly difficult, imagine trying to slow down a swinging pendulum. At first, a gentle touch removes a noticeable amount of motion. But as the pendulum slows, each additional reduction requires more precise, carefully timed interventions. Eventually, the pendulum barely moves at all, and stopping it completely becomes nearly impossible without interfering at a fundamental level.
Cooling matter works in a similar way. At high temperatures, particles are energetic and chaotic. Removing energy is relatively easy. But at very low temperatures, particles occupy only the lowest available energy states. There are fewer ways for them to give up energy, fewer interactions that allow heat to escape.
As the temperature drops, heat capacity decreases. This means that removing the same amount of energy produces smaller and smaller changes in temperature. The system becomes stubbornly resistant to further cooling.
This resistance is not a mechanical obstacle but a statistical one. It arises from the way energy levels are distributed and populated in matter. Nature does not allow an easy shortcut to absolute zero.
Quantum Mechanics and the Persistence of Motion
Even if we could overcome the thermodynamic barriers, quantum mechanics introduces an even deeper reason why absolute zero cannot truly be reached. In the quantum world, the idea of complete stillness is an illusion.
According to quantum mechanics, particles cannot have both a perfectly defined position and a perfectly defined momentum at the same time. This principle, known as the uncertainty principle, means that particles can never be completely at rest. Even in their lowest energy state, they retain a residual motion known as zero-point energy.
Zero-point energy is not thermal energy in the classical sense, but it is still motion. Atoms continue to vibrate, fields continue to fluctuate, and space itself is never truly calm. Absolute zero does not eliminate motion; it only removes all removable energy.
This quantum restlessness means that the classical image of atoms frozen motionless at absolute zero is fundamentally incorrect. The universe refuses to be perfectly still. Motion is not just a consequence of heat; it is a built-in feature of reality.
The Strange Behavior of Matter Near Absolute Zero
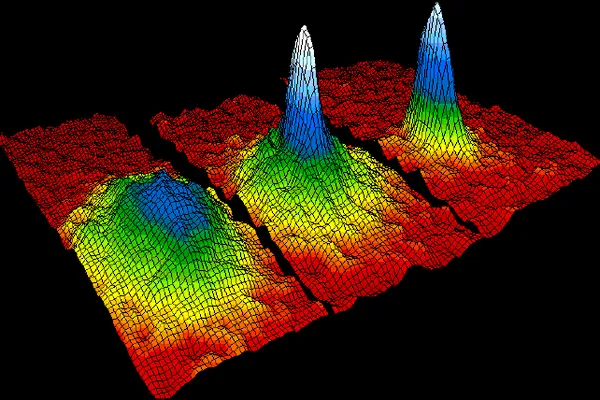
As physicists have learned to cool matter to astonishingly low temperatures, they have discovered behaviors that feel almost magical. Near absolute zero, the familiar rules of everyday experience break down, revealing a world governed by quantum effects on a macroscopic scale.
Superconductivity emerges when certain materials lose all electrical resistance, allowing current to flow indefinitely without energy loss. Superfluidity appears when liquids flow without viscosity, creeping up walls and passing through microscopic pores without friction. These phenomena are not anomalies. They are direct consequences of approaching the lowest energy states allowed by nature.
These behaviors illustrate a crucial point. Approaching absolute zero does not merely make things colder. It transforms the nature of matter itself. Systems reorganize into coherent quantum states that would be impossible at higher temperatures.
Yet even in these extraordinary conditions, absolute zero remains unattainable. The closer we get, the more nature reveals its complexity, not its simplicity.
The Role of Entropy and the Arrow of Cooling
Entropy is one of the most misunderstood concepts in physics, often associated vaguely with disorder. In reality, entropy measures the number of ways a system can arrange its internal energy while appearing the same from the outside.
At high temperatures, systems have many available configurations. Energy can be distributed in countless ways. As temperature decreases, the number of accessible configurations shrinks. Near absolute zero, only a few arrangements remain possible.
Reaching absolute zero would require compressing the system into a single, perfectly ordered state. But doing so would require infinite precision and infinite time. Any real process introduces imperfections, fluctuations, and disturbances that prevent perfect ordering.
Entropy does not forbid low temperatures. It forbids perfect finality. It ensures that the last step to absolute zero is always beyond reach.
Cooling Techniques and Their Fundamental Limits
Over the past century, scientists have developed remarkably sophisticated methods to cool matter. Cryogenic techniques, laser cooling, evaporative cooling, and magnetic trapping have pushed temperatures to billionths of a kelvin above absolute zero.
Each method relies on clever ways of removing energy. Laser cooling uses light to slow atoms. Evaporative cooling allows the most energetic particles to escape, lowering the average energy of those that remain. Magnetic fields manipulate quantum states to extract heat.
Yet all of these methods eventually encounter the same wall. As the temperature drops, efficiency plummets. The processes slow down. The remaining energy becomes harder and harder to extract. The final fraction of a kelvin demands disproportionate effort.
This convergence of independent techniques toward the same limit is not coincidence. It is evidence that absolute zero is not blocked by technology but protected by physics.
Why Infinite Time Would Still Not Be Enough
One might imagine that with infinite patience, absolute zero could eventually be reached. But even infinite time does not solve the problem. The third law of thermodynamics is not a statement about practical difficulty. It is a statement about logical impossibility.
To reach absolute zero would require a process with perfect efficiency, no losses, and no fluctuations. Such a process cannot exist in a universe governed by quantum uncertainty and thermodynamic constraints.
Even the act of measurement introduces energy. Even isolation is imperfect. Even waiting forever does not bypass the structure of physical law. Absolute zero is not delayed; it is forbidden.
Absolute Zero and the Illusion of Completion
There is something psychologically powerful about the idea of absolute zero. It feels like completion, like reaching the end of a scale. But physics teaches us that nature rarely offers endpoints in such a simple way.
Just as the speed of light cannot be reached by objects with mass, absolute zero cannot be reached by systems with entropy. These limits are not accidents. They are part of the deep architecture of reality.
They tell us that nature values continuity over closure, approach over arrival. The universe allows us to get closer and closer to its boundaries, but never to cross them. There is always another layer of complexity, another constraint, another mystery waiting at the edge.
The Philosophical Meaning of an Unreachable Temperature
Beyond equations and experiments, the impossibility of reaching absolute zero carries philosophical weight. It challenges our instinct to believe that every goal can be achieved with enough effort. It reminds us that some limits are not obstacles to overcome but truths to understand.
Absolute zero represents the idea that perfection, in the physical sense, is unattainable. Complete stillness, total order, infinite precision—these are ideals, not realities. The universe operates in gradients, not absolutes.
This realization does not diminish science. It enriches it. It shifts the focus from conquest to comprehension, from control to curiosity. Physics does not promise ultimate mastery. It offers deeper understanding.
What Absolute Zero Teaches Us About the Universe
The fact that absolute zero cannot be reached tells us that motion is fundamental, not optional. Energy, uncertainty, and fluctuation are not imperfections in the universe; they are essential features.
It tells us that the laws of physics are not just descriptive but prescriptive. They do not merely report what happens; they define what can happen. Absolute zero is not missing from reality. It is excluded by design.
It also tells us that the universe is generous in one sense and strict in another. It allows extraordinary phenomena to emerge near absolute zero, revealing hidden layers of order and coherence. But it refuses to grant the final step, the ultimate stillness.
The Emotional Power of an Unreachable Limit
There is a quiet poetry in the fact that absolute zero cannot be reached. It mirrors the human experience of striving toward ideals that guide us but remain forever out of reach. We aim for perfection, knowing we will never fully attain it, yet finding meaning in the pursuit itself.
Scientists who work near absolute zero often describe their work with a sense of reverence. They know they are approaching one of nature’s deepest boundaries. Each fraction of a degree feels like a small victory, not because it brings completion, but because it reveals something new.
In this way, absolute zero becomes less a failure and more a promise. It promises that the universe is deeper than our ambitions, richer than our goals, and more subtle than our intuitions.
Why Absolute Zero Will Always Remain Beyond Our Grasp
Absolute zero cannot be reached because temperature is not something that can simply be dialed down to nothing. It is woven into the structure of matter, energy, and information. Thermodynamics blocks the path, quantum mechanics dissolves the idea of complete stillness, and entropy ensures that the final step is always missing.
This is not a limitation of imagination or ingenuity. It is a feature of reality itself. Absolute zero is not waiting for us somewhere far ahead. It is a boundary that defines the space in which physics unfolds.
The Beauty of a Universe With Limits
In the end, the impossibility of reaching absolute zero is not a disappointment. It is a reminder that the universe is not arbitrary. It is governed by principles that balance possibility and constraint, freedom and order.
Physics does not exist to grant us ultimate control. It exists to reveal the patterns that shape existence. Absolute zero stands as one of those patterns, a silent marker of how far we can go and no further.
And perhaps that is its greatest lesson. The universe invites us to explore, to approach, to understand, but not to finish. There will always be a horizon just beyond reach, urging us forward, not to conquer it, but to learn from the journey toward it.