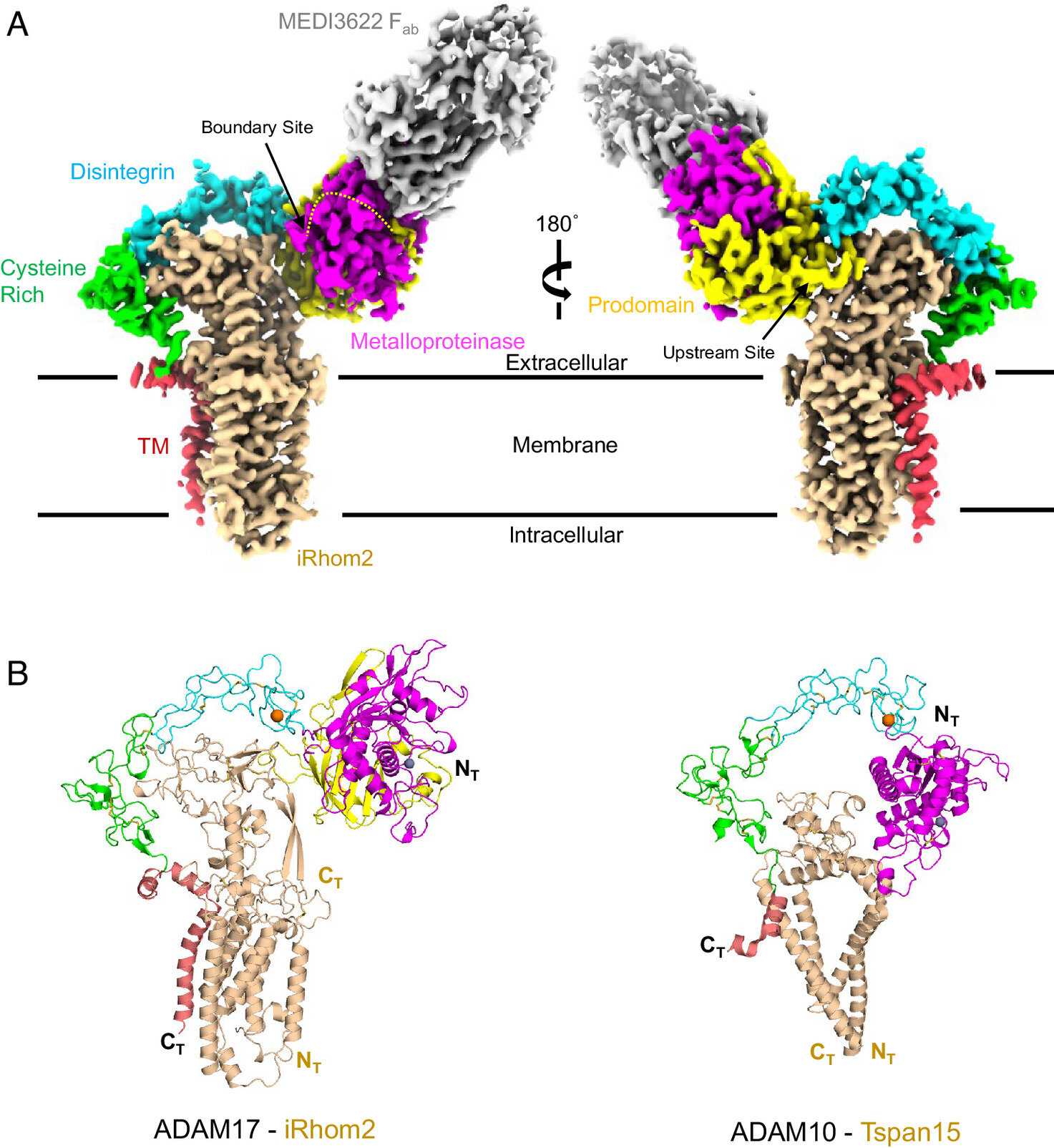
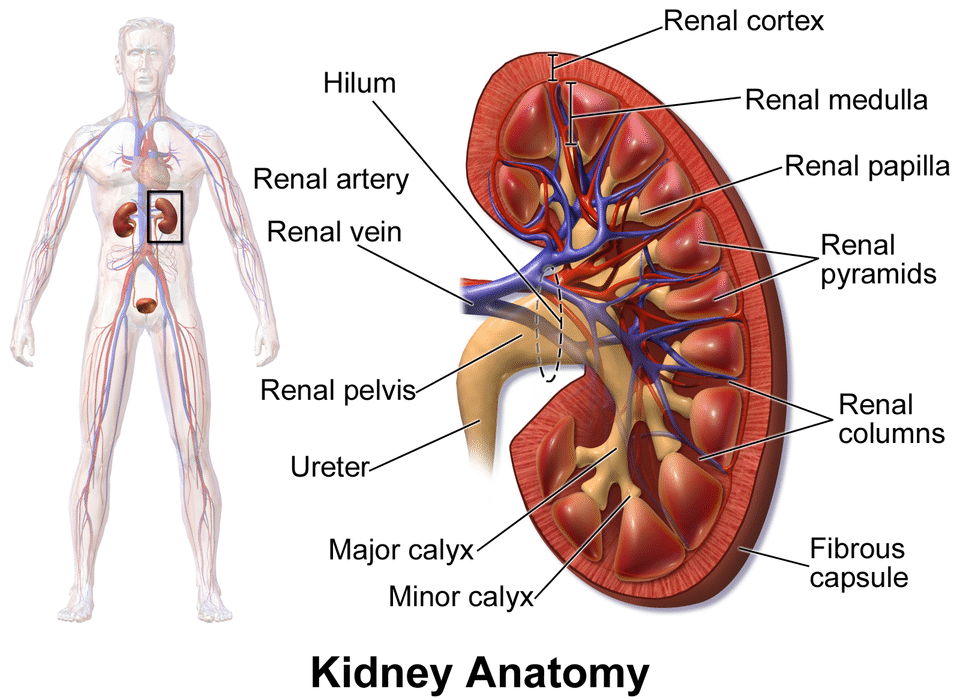
For the first time, scientists are seeing what was once invisible. Inside the human body, deep within every cell, there lies a microscopic gatekeeper—an enzyme called ADAM17—responsible for releasing chemical signals that guide immune responses, repair tissue, and, when malfunctioning, ignite chronic diseases. Now, thanks to cutting-edge imaging technology at the University of Cincinnati, researchers have finally captured a glimpse of this enzyme’s intricate structure and its critical partner, a protein known as iRhom2.
The discovery, published this week in the Proceedings of the National Academy of Sciences, marks a breakthrough moment in structural biology and offers new hope for the future of targeted therapies. For diseases like cancer, COVID-19, and rheumatoid arthritis—where immune regulation goes awry—this research could be a turning point.
“If you can see something, you can figure out how it works,” said Dr. Tom Seegar, the lead scientist behind the study, Ohio Eminent Scholar, and assistant professor at UC’s Department of Molecular and Cellular Biosciences. “We are figuring out what this enzyme looks like and how it’s regulated.”
It’s more than just seeing—it’s understanding. And understanding could lead to life-saving solutions.
A Microscopic World Made Visible
In UC’s newly launched Center for Advanced Structural Biology, inside a humming laboratory filled with cold, powerful machines, Seegar’s team harnessed cryogenic electron microscopy (cryo-EM) to peer into the molecular machinery of the human body. This technology—revolutionary in its clarity—freezes proteins mid-motion and photographs them in atomic detail. Using this method, researchers reconstructed the three-dimensional structure of the ADAM17-iRhom2 complex for the first time.
The enzyme ADAM17 is no ordinary protein. It’s a molecular switch that, when flipped, slices off signaling molecules from cell surfaces and sends them out to orchestrate biological responses. It’s crucial for life—without it, embryos can’t develop and immune defenses falter. But in disease, that switch can get stuck in overdrive.
“In some cancers and in rheumatoid arthritis, we’re seeing way too much signaling,” said Joe Maciag, Ph.D., a research scientist in the Seegar Lab and one of the study’s lead authors. “But current treatments can blunt the immune system so severely that they cause worse side effects than the disease itself.”
That’s where iRhom2 enters the scene. As a chaperone and regulator for ADAM17, it acts like a molecular steering wheel, controlling when and where the enzyme activates. Until now, no one had ever visualized how these two complex molecules fit together—or how iRhom2 sends instructions from within the cell to regulate what ADAM17 does outside of it.
By visualizing their interaction, Seegar’s team revealed an elegant and delicate dance between two molecular giants. At the heart of their discovery: a tiny segment of iRhom2 known as the “re-entry loop,” a structural feature that transmits intracellular signals to the enzyme’s outer surface. This discovery could become a crucial target for future drugs—allowing scientists to fine-tune immune responses without silencing the immune system entirely.
From Atoms to Answers
Seegar’s lab, supported by the Advanced Research Computing Center at UC, processed enormous volumes of data to reconstruct the ADAM17-iRhom2 complex. They relied on UC’s high-powered cryo-EM microscope—an advanced transmission electron microscope installed in 2022—capable of rendering proteins in exquisite detail.
“Having this microscope on campus is a game-changer,” Maciag said. “It’s allowing us to solve important biological structures without ever leaving UC. That’s not just convenient—it’s transformational.”
It’s also the first peer-reviewed paper to emerge from UC’s Center for Advanced Structural Biology, a facility built to explore biology at its most fundamental level. For researchers like graduate student Conner Slone, co-author of the study, it’s an opportunity to explore uncharted territory.
“These adapter proteins like iRhom2 are not well understood,” Slone said. “Our goal now is to understand them better and identify precise control points. If we can control these molecules, we may be able to control entire disease states.”
The Human Impact
Behind every molecular image and every line of data lies a human story. For patients battling autoimmune disorders, for families confronting cancer diagnoses, for the millions who suffered through COVID-19’s severe inflammation—this discovery matters.
ADAM17 is implicated in nearly all of these conditions. It’s involved in releasing TNF-alpha, a potent immune signal tied to inflammation. In diseases like rheumatoid arthritis, too much TNF-alpha leads to tissue destruction and chronic pain. In cancer, ADAM17 may help tumors grow by enabling them to evade the immune system. And in severe COVID-19, it plays a role in the “cytokine storm” that can overwhelm the body.
Current therapies, like TNF inhibitors, blunt these responses—but they can leave patients vulnerable to infection and other complications. Targeting iRhom2, the regulatory partner of ADAM17, might offer a gentler, more selective approach.
“This work provides a foundation for designing therapies targeting ADAM17-related diseases,” Seegar said. “We’re offering new strategies to address critical health conditions—not just at the surface level, but at the source.”
A New Era for Structural Biology
The story of this discovery isn’t just about one enzyme or one disease. It’s about what’s now possible.
Structural biology—the science of mapping proteins in 3D—has been reborn with cryo-EM. What once required years of effort using crystallography can now be done in months or even weeks. With every protein imaged, scientists get closer to understanding the language of life: how molecules fold, how they function, how they misfire—and how they can be fixed.
“This is the start of something much bigger,” Seegar said. “With the tools we have now, we can solve protein structures that were once considered impossible. We can ask questions we didn’t know how to ask before.”
Backed by state-of-the-art facilities and a growing network of researchers, UC’s Center for Advanced Structural Biology is poised to lead in this new frontier. And with discoveries like the ADAM17-iRhom2 complex, it’s already proving that big answers often start with small, invisible parts.
Looking Ahead
The Seegar Lab’s work is just beginning. With the molecular blueprint of ADAM17 and iRhom2 now in hand, researchers will dive deeper—exploring how these proteins interact with others, how mutations might alter their behavior, and how new therapies could be crafted with laser precision.
But for now, the team takes a moment to reflect on what they’ve seen: not just the elegant architecture of proteins, but the possibilities they unlock.
“It’s thrilling to be part of this,” Maciag said. “We’re helping decode how inflammation works at the molecular level. That’s going to change how we treat disease.”
And it all started with the simple act of seeing what was once unseen.
Reference: Joseph J. Maciag et al, Structural insights into the activation and inhibition of the ADAM17–iRhom2 complex, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2500732122