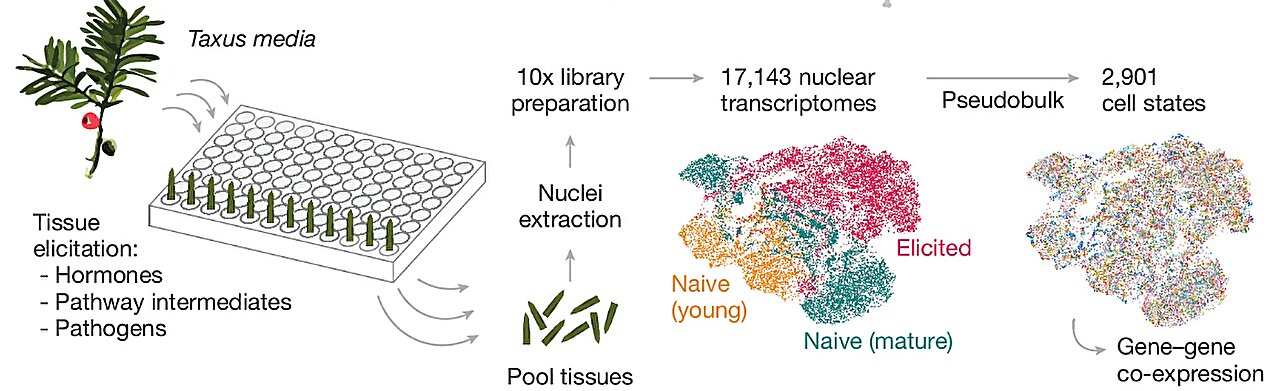
In a breakthrough that could transform the global supply chain of one of the world’s most vital cancer drugs, scientists at Stanford University have uncovered eight previously unknown genes involved in the biosynthesis of baccatin III—a crucial precursor to the chemotherapy agent paclitaxel, better known as Taxol.
The discovery, published in Nature, marks a long-awaited milestone in the decades-long quest to recreate the complex natural chemistry of yew trees in a laboratory setting. Using advanced single-cell gene mapping techniques and experimental gene expression in tobacco leaves, the research team successfully reconstructed the baccatin III pathway with levels comparable to those found in yew needles. Their achievement may finally offer a sustainable and scalable alternative to the slow, destructive process of harvesting yew trees for medicine.
The Natural Bottleneck of a Life-Saving Drug
Paclitaxel has saved countless lives since its introduction in the 1990s. It works by stabilizing microtubules in rapidly dividing cells, disrupting cancer’s ability to multiply. But its natural source—the bark of Taxus species yew trees—is rare, slow-growing, and dangerously overharvested.
Extracting paclitaxel directly from yew bark is like squeezing water from a stone. The bark contains minuscule amounts of the compound—just 0.001 to 0.05% of its dry weight—and harvesting it usually kills the tree. The industry standard involves extracting the more abundant baccatin III from needles or younger shoots and chemically converting it into paclitaxel in the lab. Yet even this semi-synthetic approach remains inefficient and environmentally taxing.
For decades, researchers have tried to build a complete biological blueprint of Taxol biosynthesis. They succeeded in mapping the first dozen steps of the pathway by 2006—but then progress hit a wall. The remaining enzymes proved elusive, hidden in the vast and complex genome of the yew.
Now, the wall has cracked open.
Cracking the Yew Genome with Single-Nucleus Precision
To find the missing links, the Stanford team turned to a powerful new technique they developed called mpXsn—short for multiplexed perturbation × single-nucleus transcriptomics.
Using this method, they isolated and sequenced more than 17,000 single-nucleus transcriptomes across 272 samples from Taxus media needles. These samples spanned different tissue types, growth stages, and conditions—essentially capturing the full symphony of gene activity as the tree performed its natural chemical alchemy.
This massive dataset was then compressed into 2,901 “pseudo-bulk” transcriptional states—an innovative approach that allowed researchers to detect subtle, cell-type-specific gene activity that would otherwise be lost in traditional bulk sequencing.
From this, the scientists zeroed in on the most promising gene candidates. Then came the crucial test: would these mysterious genes function outside their native plant?
Tobacco Leaves as Living Laboratories
To answer that, researchers turned to an old ally in plant biotechnology: Nicotiana benthamiana, a species of tobacco often used for experimental gene expression. By temporarily expressing the candidate genes in tobacco leaves and analyzing the resulting metabolites with gas chromatography–mass spectrometry and nuclear magnetic resonance, they confirmed the function of eight previously uncharacterized genes essential to baccatin III biosynthesis.
Among them was a nuclear transport factor 2–like protein called FoTO1, which fine-tunes the very first oxidation step in the pathway. Other newly identified enzymes filled long-missing gaps in the transformation of early precursors into baccatin III. Intriguingly, two enzymes independently evolved to perform the same catalytic function—an example of evolutionary redundancy that may explain why the pathway has been so difficult to reconstruct.
“The identities of the new Taxol genes shed light on why they remained unknown for so long,” the authors wrote. “Pathway reconstitution required unanticipated gene families and functionalizations.”
Toward a Sustainable Future for Paclitaxel
The implications of this discovery are enormous. By reconstructing the natural route to baccatin III in a fast-growing and easily engineered plant like tobacco, researchers have laid the foundation for a future where paclitaxel can be bio-manufactured sustainably—without killing yew trees, without reliance on scarce forest resources, and potentially at a fraction of the current cost.
“Paclitaxel has long been a symbol of both medical triumph and ecological strain,” said a researcher close to the study. “This work could finally tip the balance toward sustainability.”
The newly mapped genes also offer a promising starting point for engineering microbial or yeast-based systems that could mass-produce paclitaxel precursors in bioreactors, much like insulin or antibiotics are made today.
Such an approach would not only reduce the environmental impact of drug production but also enhance supply security—particularly vital for low- and middle-income countries where access to chemotherapy can be a matter of life and death.
Beyond Taxol: A New Frontier in Plant Genomics
Even beyond paclitaxel, the real legacy of this research may be the mpXsn platform itself. In sequencing terms, most plant genomes are wild jungles—vast, repetitive, and poorly understood. Genes for specialized metabolites are rarely grouped in tidy clusters the way they are in bacteria, making functional discovery in eukaryotes an epic challenge.
By capturing the nuanced behavior of genes across thousands of single-cell contexts, mpXsn offers a way through the thicket. The approach doesn’t just map which genes are present—it reveals how they behave in the living organism, how they talk to each other, and what roles they play in complex biochemical conversations.
That makes mpXsn a potential game-changer not just for Taxol, but for a host of other plant-derived compounds with therapeutic potential—many of which remain locked in the genomes of obscure or difficult-to-grow species.
“In a way, we’re learning to listen to what plants are telling us,” said one of the study’s co-authors. “The signals have always been there—we just didn’t have the tools to hear them clearly.”
A Victory for Patience, Precision, and Plant Science
This discovery is the culmination of years—if not decades—of effort across disciplines: botany, genetics, bioinformatics, chemistry, and biotechnology. It’s a reminder of how slow and complex nature’s recipes can be, and how intricate the challenge of mimicking them in the lab.
But it’s also a victory for innovation. By combining the precision of single-nucleus transcriptomics with the power of synthetic biology, Stanford researchers have brought us closer to a future where lifesaving medicine grows not in ancient, irreplaceable trees, but in scalable, sustainable systems.
And somewhere in a quiet greenhouse, under the bright grow-lights, a tobacco plant hums with the ancient chemistry of the yew—a green laboratory for the future of cancer treatment.
Reference: Conor James McClune et al, Discovery of FoTO1 and Taxol genes enables biosynthesis of baccatin III, Nature (2025). DOI: 10.1038/s41586-025-09090-z