It’s a bit like watching a ghost pass through walls—an invisible entity slipping past barriers, seemingly defying the laws of nature. Only, in this case, it’s not a ghost; it’s a hydrogen atom, one of the most common elements in the universe. And the barrier isn’t just a wall, but an energy threshold that the atom shouldn’t technically be able to cross, at least not by traditional means.
This strange, almost magical behavior is known as quantum tunneling—a phenomenon where particles like hydrogen atoms can pass through obstacles they would ordinarily be unable to surmount. While this may sound like the stuff of science fiction, it’s a real, measurable process that could revolutionize industries ranging from energy storage to electronics. And thanks to a pioneering study from researchers at the University of Tokyo, we now have a clearer view of how this remarkable process works at the atomic level.
Hydrogen’s Quantum Dance in Palladium
Hydrogen is no ordinary atom. At low temperatures, it behaves less like a solid, predictable particle and more like a wave—a shimmering, oscillating entity that can, in some cases, slip through barriers that seem insurmountable. This wave-like behavior enables the atom to “tunnel” through energy barriers, bypassing them without the need for extra energy.
But capturing this fleeting, wave-like behavior of hydrogen atoms is no simple feat. Hydrogen is incredibly small, which makes it notoriously difficult to study. Traditional methods like X-rays or electron beams, which are typically used to observe atomic behavior, don’t work well for hydrogen because its small size means it has a very small cross-section. In other words, hydrogen doesn’t interact with these probes in a way that makes its presence obvious.
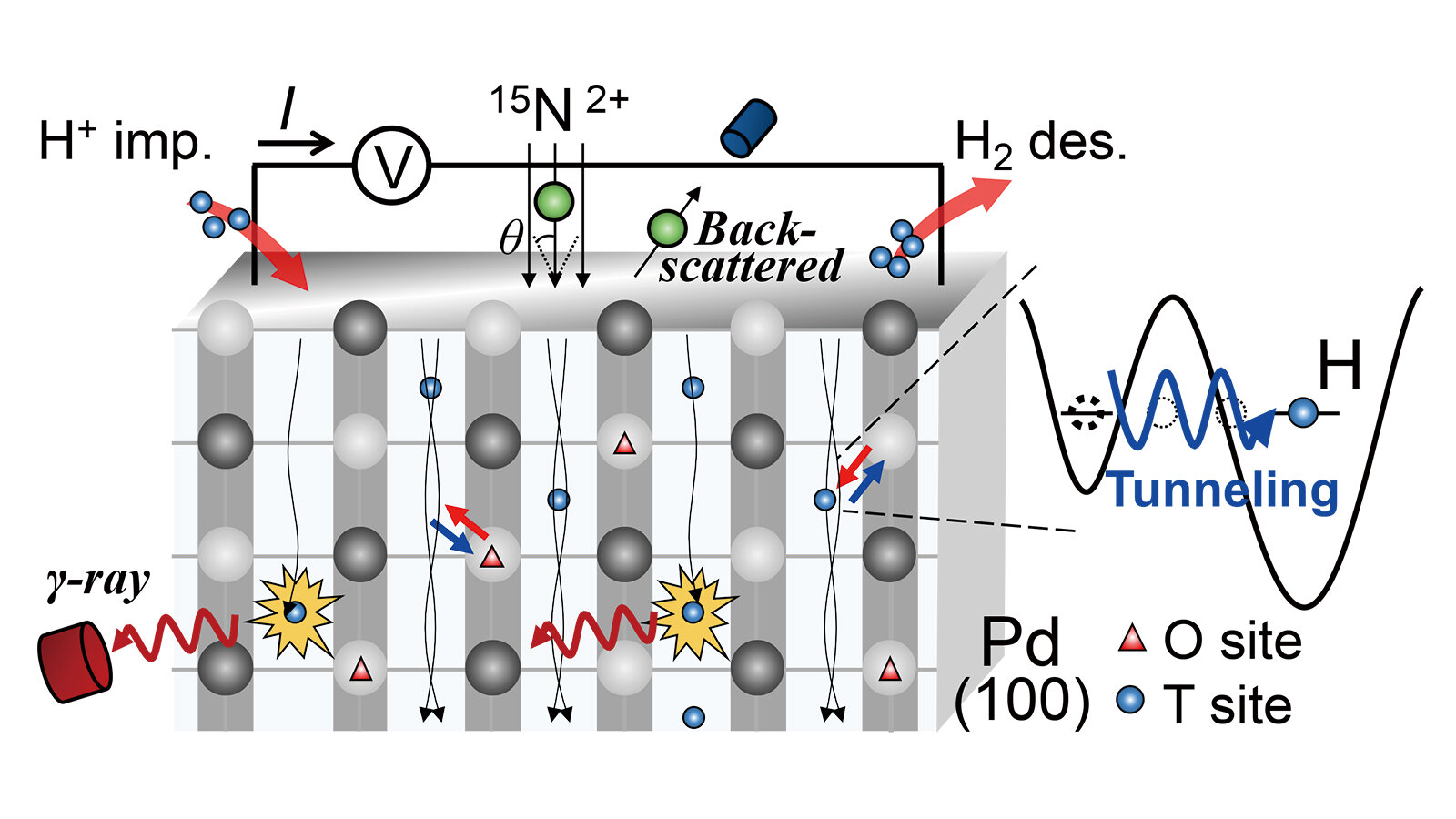
Enter the team from the Institute of Industrial Science at The University of Tokyo, who managed to observe hydrogen’s quantum tunneling behavior in a substance known as palladium—a metal that has a remarkable ability to absorb hydrogen. The researchers used a specialized technique called channeling nuclear reaction analysis, which allowed them to detect hydrogen atoms with precision in the palladium lattice. And what they found was nothing short of groundbreaking.
A Metal That Hosts the Quantum Leap
Palladium is a metal that has an intriguing property: it can absorb hydrogen atoms into its atomic lattice, where the hydrogen occupies spaces between palladium atoms. These spaces, known as interstitial sites, come in two shapes—octahedral and tetrahedral. The octahedral sites are stable and provide a stable home for the hydrogen atom, while the tetrahedral sites are less stable, offering a temporary resting place.
At higher temperatures, hydrogen atoms have enough energy to move, or hop, from one interstitial site to another. But at low temperatures, things get more interesting. Instead of simply waiting for enough energy to break through the barrier between the sites, hydrogen atoms use quantum effects to tunnel through the energy barrier. This is where things get really fascinating: in the world of quantum mechanics, energy barriers are not always as solid as they seem. In certain conditions, particles can pass through them as if the barrier doesn’t even exist.
As Takahiro Ozawa, the lead author of the study, explains, “To understand the quantum nature of hydrogen, we need to identify the hopping pathway.” But how do you observe an atom so small that it doesn’t interact with conventional probes? The researchers found their answer in channeling nuclear reaction analysis, a method that allowed them to pinpoint the exact locations of hydrogen atoms as they moved through the palladium lattice.
The Quantum Path of Hydrogen
The study revealed that when hydrogen atoms are injected into palladium, they initially occupy the metastable tetrahedral sites—those temporary resting spots. From there, they tunnel through the energy barrier to the more stable octahedral sites. The team was able to measure the rate at which this tunneling occurred by observing the changes in electrical conductivity in the palladium. This provided a key clue about the hydrogen atoms’ movement.
As the researchers heated the palladium, they noticed something interesting: above 20 Kelvin, the tunneling rate increased slightly with temperature. This is a clear indication that phonons—the vibrations of the palladium lattice—were playing a role in the tunneling process. Phonons, in a way, help guide the hydrogen atom along its path, like a series of tiny vibrations pushing the atom through the barrier.
However, below 20 Kelvin, the behavior changed. The tunneling rate decreased slightly as the temperature dropped. This was a sign that the conduction electrons—the free-moving electrons in the palladium metal—were involved in the process. These electrons, however, couldn’t perfectly follow the hydrogen atoms as they moved, suggesting that they were struggling to keep pace with the tunneling process.
Katsuyuki Fukutani, the senior author of the study, added, “Above 20 K, the tunneling rate slightly increased with the temperature, a signature of phonon effects. However, below 20 K, the tunneling rate slightly decreased with the temperature, signaling the involvement of conduction electrons that could not perfectly follow the motion of the hydrogen atoms.”
Why This Discovery Matters
So, why should we care about the quantum tunneling of hydrogen in palladium? It’s more than just an interesting scientific curiosity. This discovery opens the door to a better understanding of hydrogen behavior at the atomic level, which could have far-reaching implications for technology.
For one, understanding how hydrogen atoms move through materials like palladium could improve hydrogen storage technologies. Palladium is often used in hydrogen storage and filtration systems, and by understanding the quantum dynamics of hydrogen in these systems, we could potentially develop more efficient ways to store and release hydrogen—an essential step in advancing hydrogen fuel technologies.
Moreover, this research also has applications in quantum computing. The ability to manipulate and control atomic behavior based on quantum effects is key to the development of new materials and technologies in the quantum realm. By observing how hydrogen atoms behave when subjected to quantum tunneling, scientists can gain deeper insights into the behavior of quantum particles, which could help them develop better materials for quantum devices.
Ultimately, this study doesn’t just reveal a hidden property of hydrogen. It opens a window into the larger quantum world—a world where particles don’t behave like ordinary objects and where the very rules of physics can bend in ways that we still don’t fully understand. As the team from the University of Tokyo continues to explore these quantum effects, they’re not just studying atoms—they’re pushing the boundaries of what we know about the universe itself.
More information: Takahiro Ozawa et al, Observation of proton tunneling correlated with phonons and electrons in Pd, Science Advances (2025). DOI: 10.1126/sciadv.ady8495. www.science.org/doi/10.1126/sciadv.ady8495