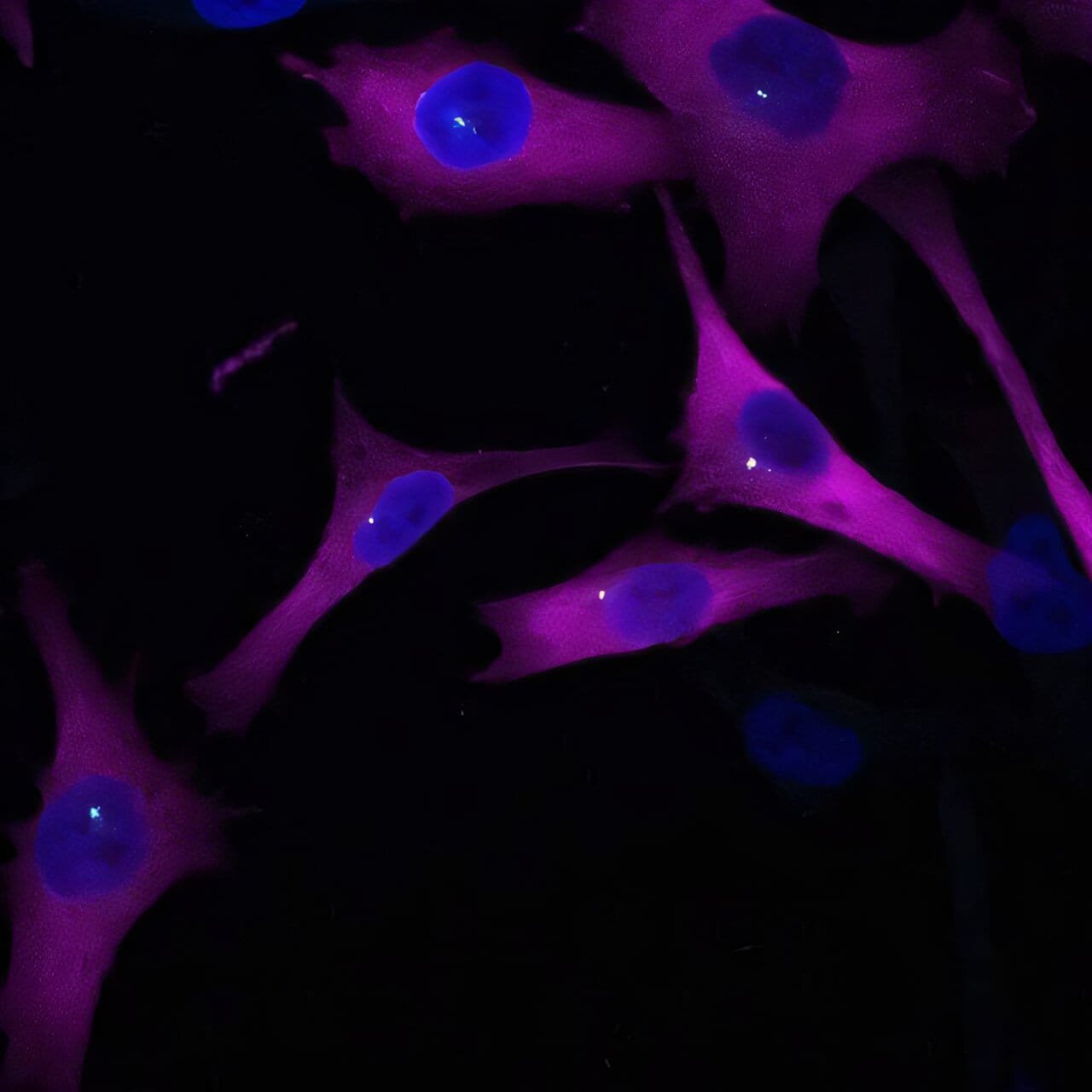
In the shadowy world of cancer metastasis, where malignant cells break free from their birthplace and travel to colonize distant organs, researchers have just illuminated a surprising new player. A protein known as eIF2A, long thought to be a quiet responder to cellular stress, has been unmasked as a critical guide steering melanoma cells as they migrate through the body.
Published in the journal Science Advances, this groundbreaking study shines a light on how melanoma—the deadliest form of skin cancer—may exploit this protein to spread with lethal precision. The discovery doesn’t just challenge previous assumptions about what eIF2A does. It opens the door to promising new ways to stop cancer in its tracks before it can metastasize, a shift that could save thousands of lives each year.
From Protein Production to Cancer’s Steering Wheel
Scientists have long believed that eIF2A operates behind the scenes, stepping in when cells are under stress to assist in launching protein synthesis. But in the context of melanoma, the story is far more insidious.
According to Dr. Fátima Gebauer, corresponding author and senior researcher at the Center for Genomic Regulation (CRG) in Barcelona, eIF2A has been hiding a second life. “Malignant cells that metastasize need to make their way through tissues in order to invade proximal or distant organs,” she explains. “Targeting eIF2A could be a new strategy to impede melanoma breaking free and seeding tumors elsewhere.”
The study’s findings indicate that melanoma cells become dependent on eIF2A for movement, not for manufacturing proteins. This revelation marks a profound shift in understanding, one that repositions eIF2A from a background molecular technician to a key navigator in cancer’s escape plan.
Melanoma: A Small Threat with a Devastating Punch
Though it makes up only a small portion of skin cancer cases, melanoma claims the lives of nearly 60,000 people globally each year. It does so not by overwhelming the skin, but by spreading—silently and lethally—to other organs. While early-stage, localized melanoma has a 99% five-year survival rate, that number plummets to just 35% once the disease has metastasized.
Preventing this transition from a confined tumor to a body-wide threat is the holy grail of melanoma treatment—and a central focus of cancer research.
Understanding how these cells orchestrate their migration could unlock new methods for trapping them before they escape. That’s where eIF2A, it turns out, may offer a rare and valuable target.
Disarming Melanoma’s Mobility
To unravel eIF2A’s secret role, the team at CRG worked with a clever model: two genetically matched human skin cell lines—identical except for one being metastatic and the other not. When they dialed down eIF2A in the metastatic cells, something dramatic happened.
The three-dimensional tumor spheres—miniature tumor models—stopped growing. The cells’ ability to move across a scratch wound in culture dishes slowed dramatically. Surprisingly, the cells didn’t show much trouble manufacturing proteins. This shattered the old assumption that eIF2A’s main job was to kickstart protein synthesis.
Clearly, eIF2A was doing something else. Something essential to movement.
To find out what, the researchers used a method not unlike molecular fishing. They “hooked” eIF2A and pulled it out of the cell, bringing along whatever other proteins it was attached to. What they discovered next was the molecular equivalent of finding a GPS unit in a thief’s getaway car.
The Centrosome: Cancer’s Cellular Compass
Among the eIF2A’s many partners were proteins from the centrosome—a structure deep inside cells that helps organize the internal scaffolding, or microtubules, used for movement. In healthy cells, the centrosome plays a key role in orienting movement, ensuring the cell knows which direction to go.
But in the cancer cells without eIF2A, the centrosome frequently pointed the wrong way. Like a compass gone haywire, the cells couldn’t steer effectively. This misdirection halted their advance, as if cutting the lines of communication between the cancer cell and its environment.
Further experiments revealed something even more surprising: it wasn’t the whole eIF2A protein, but its tail that mattered most. Trimming this tail—just a small region of the protein—disrupted cell migration. The tail, researchers now believe, acts like a scaffolding support, holding together the centrosome so it can properly guide movement.
“The tail behaves like scaffolding cement,” says Dr. Jennifer Jungfleisch, first author of the study. “It holds key parts of the melanoma’s cellular compass in place so that malignant cells can navigate their way out of the primary tumor.”
A New Target With Precision Potential
One of the most hopeful aspects of this discovery is its specificity. Normal, healthy cells don’t appear to depend on eIF2A in the same way melanoma cells do. In fact, the dependence seems to only emerge after the cells become malignant.
That’s a critical distinction. Many proposed cancer drugs fail because they affect not only tumor cells but healthy tissues, causing serious side effects. But a protein like eIF2A—whose critical role seems to “switch on” only in cancerous cells—offers a rare therapeutic window.
“In this field, many potential therapeutic targets prove either redundant or essential to normal cells,” Dr. Gebauer notes. “But the discovery of a protein that quietly makes itself indispensable only when cells become metastatic could be a rare catch. Any potential vulnerability counts.”
Indeed, while researchers caution that more studies are needed—particularly in animal models and eventually in human trials—the potential here is enormous. A drug that could disrupt eIF2A’s tail function without affecting its other roles might halt metastasis without harming healthy cells.
The Future of Metastasis Research
Metastasis remains the leading cause of death in most cancers, not just melanoma. The biological leap from a localized tumor to widespread disease involves an orchestra of changes—mobility, communication, survival, and adaptation. The discovery of eIF2A’s role in one of these functions—cellular navigation—is a major advance in our understanding of that process.
This study also underscores how much we still don’t know about the proteins we’ve long assumed to understand. eIF2A, once thought to be merely a secondary player in stress response, has emerged as a critical structural component in the engine room of cancer cell migration.
It’s a reminder that in biology, context matters. A protein’s job may vary wildly depending on the environment, the type of cell, or the stage of disease. That complexity can be frustrating—but also full of opportunity.
A Compass Disrupted, A Hope Renewed
There is something poetic about the discovery: a protein once known for its response to cellular stress now exposed as a cancer’s compass. If researchers can find a way to break that compass, they may stop deadly journeys before they begin.
For the tens of thousands who die from metastatic melanoma each year—and the millions more living with the threat of recurrence—this research offers more than insight. It offers hope. Hope that we can understand the invisible paths cancer cells take, and place meaningful roadblocks in their way.
As we learn more about how cells move, adapt, and betray their original purpose, discoveries like this remind us that the battle against cancer is fought not just with chemotherapy or surgery, but with ideas—and a relentless search for the vulnerabilities that disease cannot hide forever.
More information: Jennifer Jungfleisch et al, eIF2A regulates cell migration in a translation-independent manner, Science Advances (2025). DOI: 10.1126/sciadv.adu5668. www.science.org/doi/10.1126/sciadv.adu5668