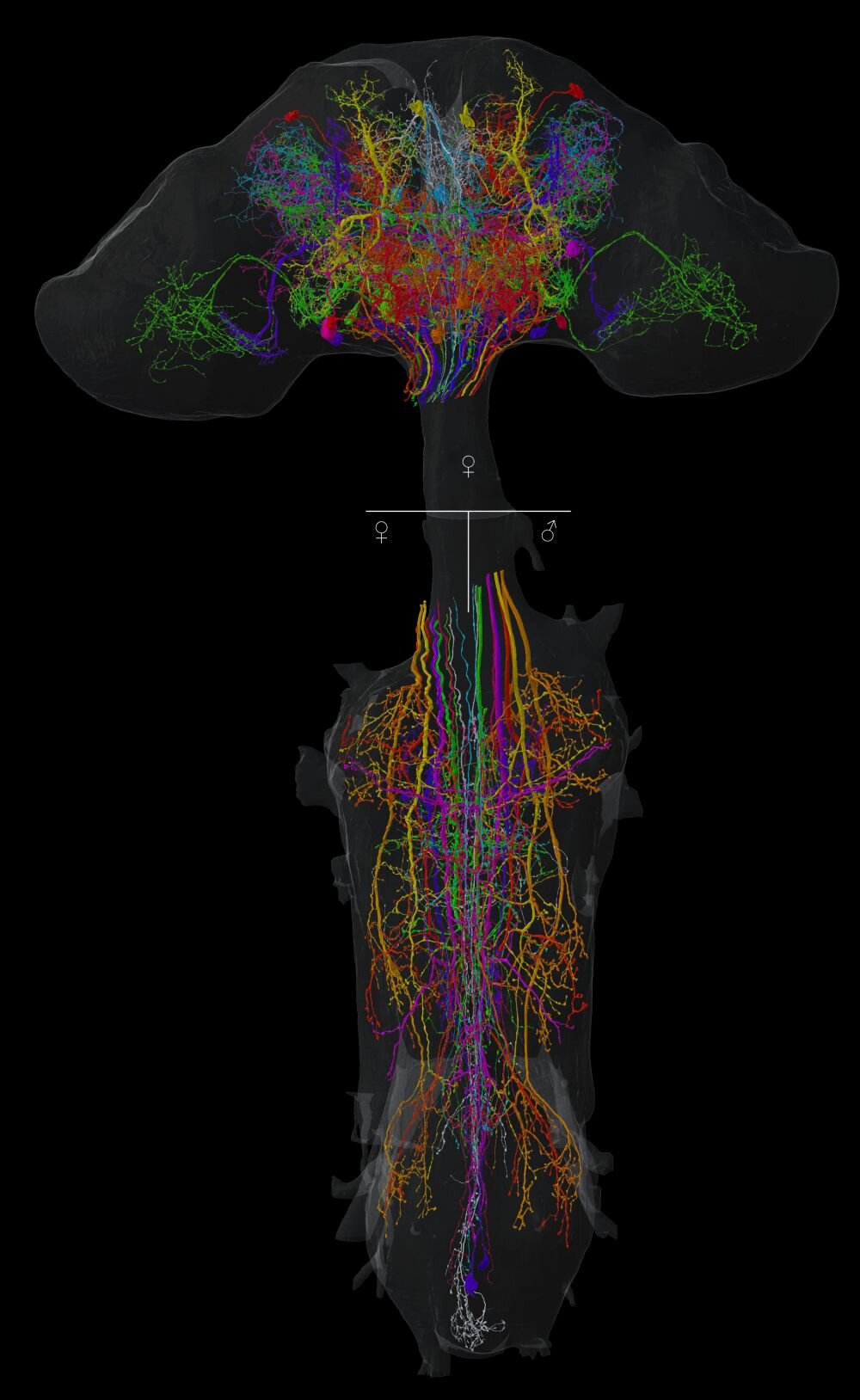
For the first time in the history of neuroscience, researchers at Leipzig University, in collaboration with international institutions, have decoded the entire nervous system of one of biology’s most powerful model organisms: the fruit fly (Drosophila melanogaster). This monumental achievement, published in the prestigious journal Nature, goes beyond mapping just the brain—it includes the entire central nervous system of both male and female flies. For decades, scientists have explored the fruit fly’s brain in slices and segments, but this new research stitches together a full, seamless map of its neural architecture. And in doing so, it rewrites our understanding of insect neurology, sexual dimorphism, and the brain-behavior relationship.
This is not merely a technical achievement. It is a revelation—a moment akin to the unveiling of a detailed world atlas after centuries of using rudimentary sketches.
Beyond the Neck: Uncovering the Missing Link
What makes this study groundbreaking is not just its completeness, but the fact that it ventures beyond the long-standing technical barrier: the neck connective. For years, connectomic maps of Drosophila stopped at the neck, failing to include the neurons that bridge the brain and the ventral nerve cord (VNC)—the insect analog of the human spinal cord. These “neck neurons” serve as the neural superhighways transmitting commands from the decision-making centers of the brain to the motor and sensory systems of the body.
“The neurons that pass through the neck connective are absolutely essential for behavior,” explains Dr. Katharina Eichler, the study’s lead researcher and a pioneer in fruit fly neuroanatomy. “Yet until now, we had no way of seeing how they connected the brain to the rest of the nervous system. These circuits were simply missing from the map.”
Using high-resolution electron microscopy, Eichler and her team have identified and traced these elusive neurons through three complete connectomes—one of a female brain and two of the ventral nerve cords (one male and one female). The implications are far-reaching: for the first time, scientists can visualize the full information pipeline that governs insect behavior from sensation to action.
The Connectome in Detail: A New Gold Standard
The fruit fly connectome is not just a map; it’s a revolution in biological detail. Comprising millions of synaptic connections among tens of thousands of neurons, the data collected represents the most comprehensive neural atlas of a complex animal to date. This is the first time all neurons of the fly’s central nervous system—both brain and VNC—have been digitally reconstructed at synaptic resolution.
The team’s analytical pipeline combined state-of-the-art electron microscopy with advanced image alignment, neuron tracing algorithms, and corroborating light microscopy data. This multidimensional approach allowed researchers not only to chart every neuron, but also to determine how these neurons form circuits, communicate, and contribute to observable behaviors.
At the core of the research is the concept of the connectome—a complete wiring diagram of an organism’s nervous system. In the same way that the human genome transformed genetics, the fruit fly connectome stands to revolutionize our understanding of how brains compute, how behavior arises, and how sex differences are encoded in neural tissue.
Sex on the Circuit Board: The First Comparative Connectome
Perhaps the most fascinating outcome of this research is its revelation of sexual dimorphism in the fruit fly nervous system. By comparing the male and female connectomes, the researchers uncovered neural structures that exist only in one sex and not the other—neurons that are fundamentally built into the fly’s blueprint for sex-specific behaviors.
For instance, the team identified a previously uncharacterized descending neuron called aSP22, which communicates with female-specific neurons in the abdominal region. When this neuron is active, females exhibit abdominal extension—likely a preparation for egg-laying. In contrast, the same neuron in males communicates with a different circuit, triggering the abdomen to curl forward in a mating gesture.
“This is the first clear neural explanation for why the same neuron produces sex-specific behavior,” says Eichler. “It suggests that behavior is not just a product of hormone levels or experience, but of fundamentally different wiring patterns.”
This discovery pushes neuroscience into a new realm where sexual identity is not only expressed through morphology or behavior, but hard-coded into the neural infrastructure itself. It opens the door to comparative studies that could reveal how evolution sculpts brains differently to produce different behaviors in males and females.
From Blueprint to Behavior: A Roadmap for the Future
Beyond its immediate scientific value, the newly published connectome acts as a navigational map for the next generation of brain research. It serves as a reference atlas—a neural GPS for biologists seeking to link neurons to behavior, to dissect circuits underlying memory, sleep, decision-making, aggression, and more.
“With a complete connectome, experiments can now be designed with surgical precision,” Eichler explains. “Instead of randomly targeting neurons or relying on broad assumptions, scientists can home in on exact circuits. That saves years of work and mountains of resources.”
This is especially important in Drosophila, where sophisticated genetic tools like optogenetics and neuron-specific promoters allow researchers to activate, silence, or modify single cells or entire networks. Now, thanks to the connectome, those interventions can be planned with anatomical certainty.
Overcoming the Technical Mountains
Creating a full connectome of an adult Drosophila was no small feat. The process required slicing the nervous system into thousands of ultrathin sections (each just nanometers thick), photographing each one with electron microscopes, and stitching the images together into a continuous three-dimensional volume. The amount of data generated was staggering—terabytes of high-resolution images requiring months of processing and meticulous manual annotation by trained neuroscientists.
Previous connectomic projects, such as the partial map of the larval fruit fly brain or even the famous full connectome of the nematode Caenorhabditis elegans, lacked the sheer complexity and behavioral relevance of this work. C. elegans, with just 302 neurons, pales in comparison to Drosophila, which has over 100,000 in its central nervous system.
Now that the technical barrier has been breached, the path is open for similar efforts in even more complex brains—possibly even vertebrates. But for now, the fruit fly remains the star of the connectomic stage, and its nervous system the best-mapped in the animal kingdom.
A Living Laboratory for Brain Research
Why the fruit fly? What makes this tiny insect the darling of neuroscience?
The answer lies in its exquisite combination of complexity and tractability. Drosophila melanogaster has been a staple of biological research for over a century, first in genetics, and more recently in neuroscience. Its nervous system is complex enough to produce rich, varied behaviors—mating rituals, decision-making, flight navigation, courtship songs—but simple enough to allow full-scale mapping and manipulation.
Moreover, fruit flies reproduce quickly, are easy to genetically modify, and have well-studied genomes and behavior repertoires. Combined with a complete connectome, these features make them unparalleled living laboratories for uncovering the rules of brain function.
The Road Ahead: Two New Connectomes and Beyond
Building on the success of the current study, Dr. Eichler’s group is now working on two new connectomic datasets—each representing the complete central nervous system of a female and a male fruit fly. These upcoming datasets promise even finer resolution, improved coverage, and deeper insights into how the nervous system evolves, functions, and fails.
Researchers anticipate that these future maps will include more peripheral circuits, sensory ganglia, and developmental annotations—essential for linking environmental inputs to internal computations and external behaviors. Eventually, these efforts may reveal how internal states (hunger, sleep, fear) shape neural activity and decision-making.
This endeavor is not just about flies. The principles uncovered in Drosophila—about how neurons wire up to perform computations, how circuits evolve, and how sex shapes brain design—could extend to higher animals, including humans.
A Final Thought: What the Fly Teaches Us About Ourselves
At first glance, the fruit fly may seem like an unlikely window into the mysteries of the brain. Yet, through this humble insect, we are now peering into the fundamental rules of cognition, behavior, and neural architecture. The new connectome is more than a technical achievement; it is a philosophical one. It reminds us that behind every twitch of a wing or curl of an abdomen lies a cascade of electrical signals, decisions, and deeply encoded patterns that shape how a living creature navigates its world.
In mapping the fly’s brain, we edge closer to understanding our own. For within those tiny synapses and circuits lie the blueprints of thought, intention, and identity. The fruit fly, long a companion in the laboratory, may well be the guide that finally illuminates the dark, tangled forest of the human mind.
Reference: Tomke Stürner et al, Comparative connectomics of Drosophila descending and ascending neurons, Nature (2025). DOI: 10.1038/s41586-025-08925-z