In a laboratory half a world apart from one another, scientists in the United States and Sweden are working on a new kind of map—a map that could transform the treatment of devastating brain diseases like Alzheimer’s and Parkinson’s. But this isn’t a map of continents or cities. It’s a map of molecules winding their way through the most mysterious terrain of all: the human brain.
And the key to building this map is something deceptively simple yet revolutionary: a molecular “click.”
Imagine snapping Lego bricks together with a satisfying click. That’s the spirit of click chemistry, the Nobel Prize-winning technique that scientists are now wielding like a precision tool to track where therapeutic molecules travel in the brain.
The Puzzle of Getting Drugs Into the Brain
The brain has long been medicine’s final frontier, fiercely protected by the blood-brain barrier—a biological fortress designed to keep out harmful substances. But that same barrier also keeps out many drugs that could help heal the brain’s delicate tissues.
Among the most promising new treatments for neurological diseases are antisense oligonucleotides—short synthetic snippets of DNA or RNA designed to bind to specific genetic instructions inside brain cells. These molecules can silence harmful genes or adjust how proteins are made.
Scientists believe antisense oligonucleotides could soon treat disorders ranging from Huntington’s disease to ALS. But there’s a problem: no one has been able to see precisely how these molecules distribute themselves inside the brain once they’re injected.
Without that knowledge, doctors are flying blind. They can’t know how much drug actually reaches diseased tissue—or whether it’s spreading evenly to where it’s needed most.
A High-Stakes Quest for Visibility
That’s where Dr. Brendon E. Cook enters the story. As a senior scientist at Biogen, a biotech company headquartered in Cambridge, Massachusetts, Cook spends his days chasing invisible molecules. His mission is to find ways to make the unseen visible.
“The determination of a drug’s biodistribution is critical to ensure that it reaches the target tissue of interest,” Cook explains. “This is particularly challenging in the brain, where invasive sampling methods may not be possible.”
The stakes couldn’t be higher. Unlocking the brain’s secrets could mean slowing—or even halting—the progression of diseases that steal memory, movement, and identity.
So Cook and his colleagues, partnering with scientists at Sweden’s prestigious Karolinska Institute, turned to one of the hottest tools in modern chemistry: click chemistry.
Click Chemistry: A Simple Reaction With Monumental Power
In 2022, the Nobel Prize in Chemistry went to Carolyn Bertozzi of Stanford University, Morten Meldal from the University of Copenhagen, and Karl Barry Sharpless, who worked at MIT and Stanford. Their achievement? Perfecting reactions that let chemists “click” molecules together as effortlessly as snapping puzzle pieces into place.
One of the crown jewels of click chemistry is the copper-catalyzed azide-alkyne cycloaddition (CuAAC), discovered independently by Meldal and Sharpless. This reaction creates a tiny molecular ring called a triazole—a bond that’s strong, precise, and remarkably clean.
For Cook and his team, click chemistry offered something priceless: a way to attach a “tracer” molecule to antisense oligonucleotides without disturbing their delicate function.
[18F]BIO-687: A Tiny Light in the Darkness
The breakthrough came in the form of a specially designed PET tracer called [18F]BIO-687. PET, or positron emission tomography, is a powerful scanning technology that can visualize tiny amounts of radioactive tracers in living tissues.
Cook’s team engineered [18F]BIO-687 to seek out antisense oligonucleotides tagged with a particular chemical handle—methyltetrazine. When the two meet inside the brain, they undergo a “click” reaction that permanently links them together.
In effect, the tracer acts like a molecular spotlight, lighting up the antisense oligonucleotides wherever they travel.
From Rats to Monkeys: A Journey Into the Brain
In their study, published in Science Translational Medicine, Cook and colleagues first tested their system in lab rats. They injected the animals with methyltetrazine-tagged antisense oligonucleotides and later introduced the [18F]BIO-687 tracer.
The results were electric—literally. PET scans revealed the tracer glowing brightly throughout the rats’ central nervous systems. Not only did the tracer find its mark, it bonded so specifically that researchers could measure exactly how much antisense oligonucleotide was present in different brain regions.
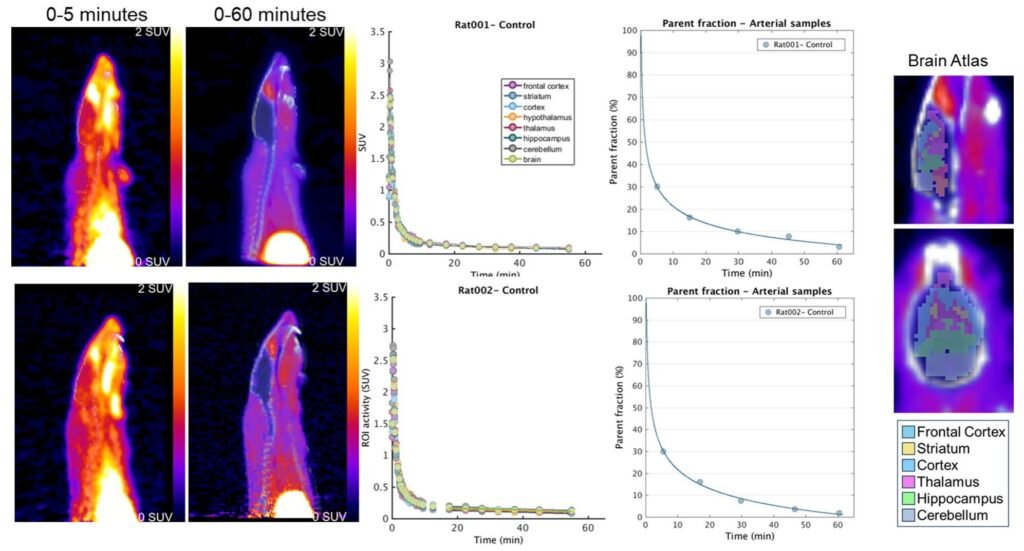
Cook explained that the PET imaging signal in the brain closely matched measurements obtained through precise laboratory techniques like liquid chromatography–mass spectrometry. This confirmed that the imaging wasn’t just showing noise—it was mapping real distributions of drug molecules.
The success didn’t stop with rodents. The same strategy was tested in non-human primates—macaque monkeys whose brains are closer to our own. The approach worked seamlessly, opening the door to future clinical studies in humans.
A second therapeutic candidate, known as BIIB080, also performed well in primates, giving researchers more confidence that the method could be widely applicable.
A Versatile Tool With Broad Implications
One of the most exciting aspects of Cook’s work is how flexible the system appears to be. The [18F]BIO-687 tracer, he says, is modular. Scientists can adapt it to bind with a variety of macromolecules—not just antisense oligonucleotides.
“The modular nature of the tracer allows for a low-profile, biocompatible modification to be applied to a wide range of macromolecules,” Cook and his colleagues concluded.
That opens a floodgate of possibilities. In the future, this technique could help scientists track the distribution of new drugs for brain cancer, psychiatric illnesses, and rare genetic conditions. It could even help researchers diagnose diseases earlier by visualizing subtle changes in brain chemistry.
The Human Dimension Behind the Science
For patients battling neurodegenerative diseases, time is a cruel adversary. Every neuron saved means memories preserved, movement restored, independence prolonged.
The scientists behind this research know that what they’re doing isn’t just chemical wizardry—it’s personal. Families waiting for a cure. Children hoping to hold onto parents slipping away into dementia. People trying to hold off the slow erosion of self that comes with diseases like ALS.
Click chemistry might seem like an abstract chemical puzzle. But for millions worldwide, it could soon become the bridge between laboratory promise and life-saving therapy.
From a magnetic “click” deep within the brain to glowing images on a PET scan, this is science at its most dazzling—and its most human.
Reference: Brendon E. Cook et al, PET imaging of antisense oligonucleotide distribution in rat and nonhuman primate brains using click chemistry, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adl1732