In a quiet laboratory on Long Island, a team of chemists may have taken a bold step toward solving one of the planet’s most urgent challenges: what to do with all the carbon dioxide we’re pumping into the atmosphere. Their weapon? A meticulously crafted molecule, designed not just to survive harsh reactions—but to transform one of Earth’s most abundant greenhouse gases into something useful, using the power of light.
This breakthrough, developed at the U.S. Department of Energy’s Brookhaven National Laboratory and published in the Journal of the American Chemical Society, converts carbon dioxide (CO₂) into formate (HCO₂⁻), an industrially valuable substance used in fuel cells, pharmaceuticals, and as an antimicrobial agent. But what makes the discovery remarkable is not just the product—it’s the process.
“This isn’t just chemistry. It’s light-powered alchemy,” said Sai Puneet Desai, lead author of the study. “We’re taking something cheap and abundant—CO₂—and adding electrons and protons, with precision, to make something useful.”
At its heart, the team’s work draws inspiration from one of nature’s greatest feats: photosynthesis.
Imitating Nature’s Genius
For over 3 billion years, plants have captured the sun’s energy and used it to transform carbon dioxide and water into sugars—fuels for life. Desai and his team aimed to replicate a piece of that elegant process, but with a modern twist. Instead of building sugar, they’re creating formate. Instead of chlorophyll, they’re using a specially engineered catalyst.
“You can think of it as storing light energy in chemical bonds,” said co-author Andressa Müller. “It’s a synthetic form of what plants do every day.”
But the story isn’t just about mimicry. It’s about mastering control—about reshaping molecules so they dance to a choreographed rhythm of electrons and protons, initiated by light.
The Catalyst That Behaves Like a Flower
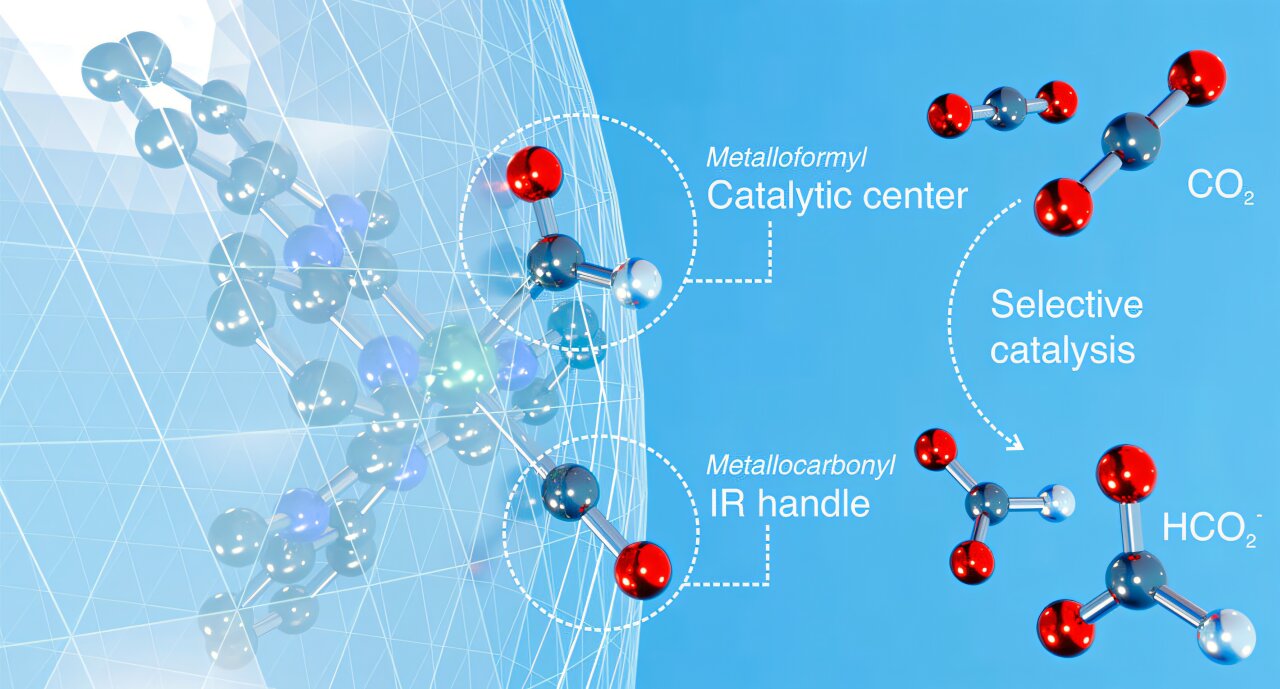
The chemical core of this innovation is a metal-based catalyst, originally centered on ruthenium. But unlike traditional catalysts that leave their metal core vulnerable to competing reactions, this one is shielded—wrapped in carefully designed ligands.
Think of the catalyst like a flower: the metal is the center, and the ligands are its petals. These ligands don’t just decorate the molecule—they control it.
“In typical CO₂ conversion processes, the metal center is exposed, which allows unwanted reactions to occur. That often leads to waste or decay of the catalyst,” said Javier Concepcion, leader of Brookhaven’s Artificial Photosynthesis group. “But with this design, every binding site on the metal is occupied. It’s locked down—protected.”
This unique ligand-based mechanism shifts the chemical action away from the metal and onto the ligands themselves. That shift changes everything.
“Because the chemistry happens on the ligands, we avoid side reactions and produce only one product: formate,” Müller explained. “No hydrogen, no carbon monoxide—just what we want.”
A Universal Blueprint
Even more exciting, this strategy isn’t limited to rare metals. While the study focused on ruthenium, the team has already begun applying the same concept to iron—a far cheaper and more Earth-abundant material.
“This paper shows that our approach is generalizable,” Concepcion said. “Our goal is to use metals like iron that are everywhere in nature. And it works.”
This opens the door to low-cost, scalable methods of carbon capture and conversion—key components in the global push for clean energy.
Building the Perfect Reaction—One Calculation at a Time
Before a single reaction occurred in the lab, the team turned to theory. Using a powerful computational tool called density functional theory (DFT), they simulated the entire process at the atomic level.
“We used electron density modeling to understand every step,” said Mehmed Ertem, Brookhaven’s resident computational chemist. “How electrons and protons move, how intermediates form, and how the catalyst evolves through the reaction.”
One of the surprises? A chemical species called a “radical cation”—an intermediate once thought too unstable to play a meaningful role—actually sticks around long enough to help drive the reaction forward.
“People assumed these radicals vanished almost instantly,” Concepcion said. “But we showed they live for hundreds of microseconds. In chemical terms, that’s an eternity.”
From Light Spark to Chemical Symphony
The system begins with a photosensitizer, a molecule that absorbs light and kickstarts the flow of electrons. These electrons are relayed to the catalyst, aided by another molecule called an organohydride, which also donates protons in a separate step.
The moment light hits the system, the photosensitizer springs into action. It channels energy, launches an electron, and sets the entire process in motion—turning CO₂ into formate in a carefully orchestrated cascade.
Crucially, once the cycle is complete, every component resets. The catalyst doesn’t wear out. The photosensitizer returns to its ground state. The system is designed for repeat performance.
“This recyclability is everything,” Desai emphasized. “We don’t want a system that generates waste. We want sustainability.”
Capturing Reactions in Real Time
To prove their theory, the team ran real-world lab experiments using advanced tools available at Brookhaven’s Chemistry Division. One of the stars of the show: the Laser Electron Accelerator Facility (LEAF), which delivers bursts of electrons and laser light with nanosecond precision.
With LEAF, scientists captured fleeting chemical intermediates in the act—like catching a lightning bolt mid-strike. They watched as radical cations formed, survived, and guided the transformation of CO₂.
“LEAF gave us a time-resolved picture of the entire reaction,” Concepcion said. “We could literally watch chemistry happen—second by second, molecule by molecule.”
They also used X-ray crystallography to map the physical structure of their catalysts and additional laser-based tools to explore other time-sensitive steps.
The combination of theory, computation, and experimental evidence painted a full picture of how light, electrons, and protons combine to bring about chemical change—with astonishing efficiency and elegance.
A Cleaner Future, Lit by Science
At a time when rising CO₂ levels threaten global stability, innovations like this one offer a measure of hope. They don’t just promise a cleaner planet; they suggest a different way of thinking about energy, chemistry, and sustainability.
Instead of treating CO₂ as waste, this research transforms it into resource. Instead of burning fossil fuels, it uses sunlight to spark useful reactions. And instead of relying on rare or expensive materials, it opens the path to chemistry grounded in the elements of Earth itself.
“This is only the beginning,” Desai said. “Our system shows what’s possible when you combine deep chemistry with smart design and clean energy. We’re just scratching the surface.”
As the world races to reimagine its energy future, the story unfolding in a small lab at Brookhaven is a vivid reminder: sometimes, the solutions to our biggest problems lie not in grand revolutions, but in tiny molecules—engineered to shine under the light.
Reference: Sai Puneet Desai et al, Photochemical Ligand-Based CO2 Reduction Mediated by Ruthenium Formyl Species, Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.5c04611