Imagine a soldier with perfect aim—but without a target. That’s the challenge the human immune system faces when battling some of the deadliest cancers. Brain, breast, and colon cancers have a notorious talent for slipping past immune defenses, cloaking themselves in biological invisibility because they originate from the body’s own tissues. But what if we could unmask these tumors, paint a bright red flag on them, and then send our immune cells in, fully trained, to seek and destroy?
That’s exactly what biomedical engineers at Georgia Tech and Emory University may have achieved. In a stunning new development published in Nature Cancer in May 2025, the team introduced a two-pronged immunotherapy strategy that could redefine how we treat some of the most stubborn cancers known to medicine.
The strategy combines synthetic biology with Nobel-winning mRNA technology and cutting-edge CAR T cell therapy to give the immune system a target it can’t ignore. The result? Lab tests showed not only the elimination of aggressive tumors—but also long-term immunity against recurrence.
This isn’t just a clever trick. It’s a potentially universal cancer treatment paradigm—and it could bring hope to patients who currently have none.
The Sneaky Nature of Solid Tumors
Unlike many blood cancers such as leukemia or lymphoma, solid tumors present a nightmarish challenge for oncologists and immunologists. These tumors are often cloaked in cellular camouflage—since they develop from normal tissue, they don’t necessarily display “foreign” signals that would alert the immune system to danger.
“They’re incredibly sneaky,” says Gabe Kwong, lead researcher on the project and associate professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University. “Tumors don’t set off alarms. They mimic healthy cells, making them nearly invisible to immune cells trained to avoid friendly fire.”
Traditional CAR T cell therapies—which engineer a patient’s own T cells to recognize and destroy cancer cells—have worked wonders in blood cancers. But applying the same method to solid tumors has been a nonstarter. Why? Because the targets (antigens) that T cells look for are often shared with healthy tissues. Attacking the tumor can mean collateral damage to essential organs. The result: toxicity, inflammation, and life-threatening side effects.
So Kwong’s team flipped the script. Instead of searching for a natural target on the tumor, they created one.
The Synthetic Antigen Breakthrough
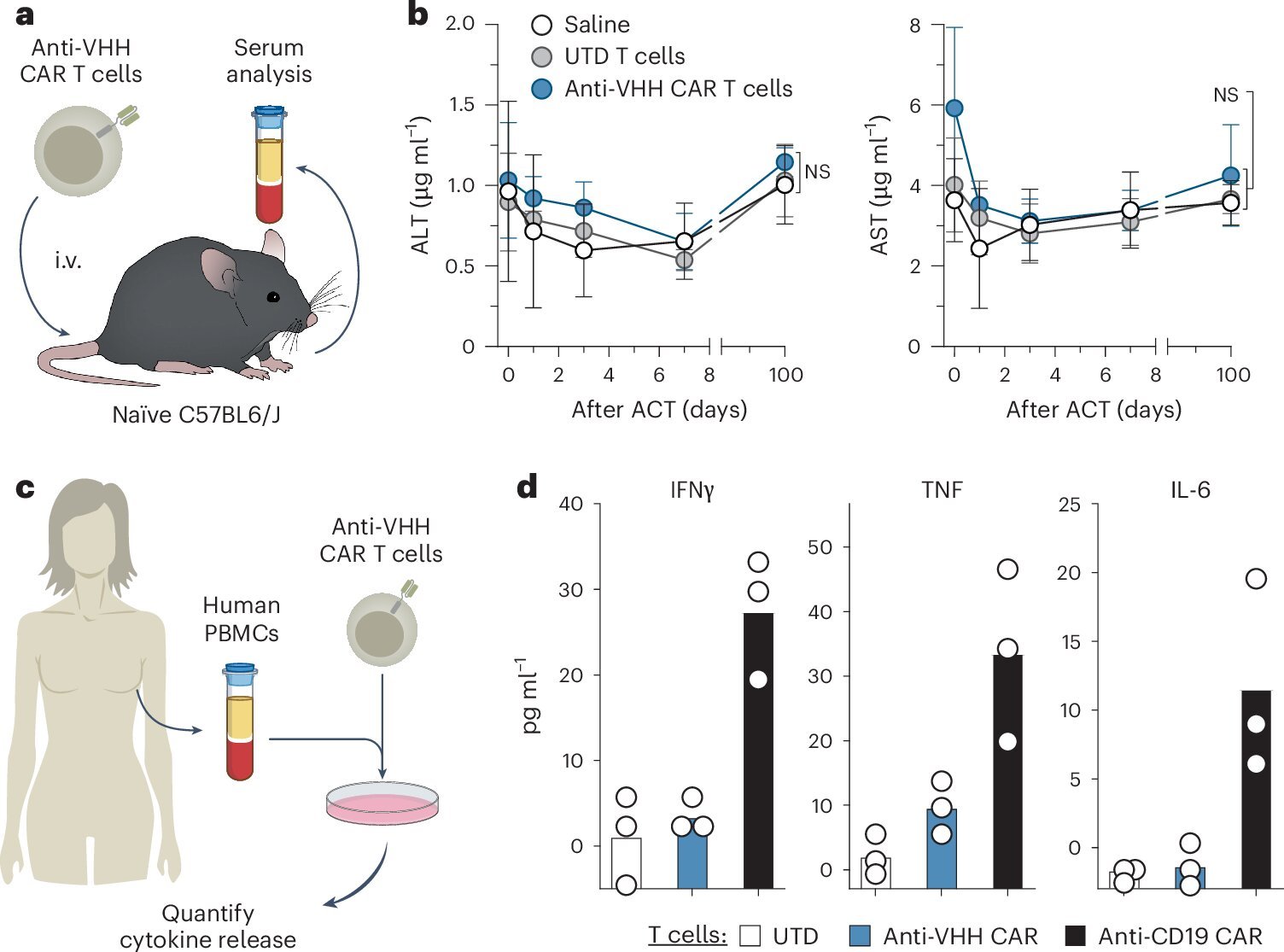
The Georgia Tech team engineered a synthetic protein antigen—one that doesn’t occur anywhere in the human body. It’s a sort of molecular name tag: foreign, conspicuous, and unmistakably artificial. Then, they used lipid nanoparticles—fatty bubbles similar to those used in COVID-19 mRNA vaccines—to deliver genetic instructions (mRNA) to tumor cells. The mRNA teaches the cancer cells to manufacture and display the synthetic antigen on their surfaces.
“Now the tumor is lit up like a flare gun,” says Kwong. “It becomes highly visible to the immune system.”
But the second piece of the puzzle is what makes this approach revolutionary. The team simultaneously engineered CAR T cells to recognize this synthetic antigen. These supercharged immune cells are then reinfused into the patient’s body. Unlike conventional therapies that struggle to distinguish tumor from healthy tissue, these CAR T cells are laser-focused: they only recognize and attack cells displaying the synthetic marker.
Training the Immune System for Long-Term Memory
What the researchers discovered next was even more exciting. After the synthetic antigen-marked tumors were eliminated, the animals were re-exposed to cancer cells—simulating a relapse. The immune system didn’t hesitate. It remembered.
“We basically turned the tumor into an immune training center,” explains Lena Gamboa, co-lead author of the study and now a research engineer at Georgia Tech. “It wasn’t just about killing cancer the first time. We taught the body how to recognize the threat if it ever returned.”
This discovery touches on one of cancer’s most insidious traits—its ability to come back. Patients who undergo surgery, chemotherapy, or radiation may achieve remission, only to face recurrence months or years later. In this study, the engineered immune response created a kind of cellular memory, providing a durable shield against relapse.
In the words of Kwong: “We’re not just creating a treatment. We’re training an immune army.”
A Universal Approach to a Diverse Enemy
One of the greatest challenges in cancer immunotherapy is the sheer heterogeneity of tumors. A therapy that works on one subtype of breast cancer may be useless against another. Cancers constantly evolve, changing their molecular features to evade drugs and immune attacks.
But this new strategy could cut through that complexity. Because the synthetic antigen is artificially introduced and doesn’t rely on naturally occurring tumor markers, it bypasses the need to identify what makes each cancer unique.
“It’s a platform, not just a therapy,” says Ali Zamat, co-lead author alongside Gamboa. “This approach could, in theory, be applied to any localized tumor that can be directly injected with the mRNA.”
This is especially significant for triple-negative breast cancers—a form that lacks the estrogen, progesterone, and HER2 receptors that most breast cancer drugs target. These patients often face chemotherapy as their only option, a treatment with severe side effects and modest success rates.
“Instead of searching endlessly for a new druggable target, we’re creating one,” Zamat explains. “We’re giving the immune system something to lock onto.”
Built on Proven, Safe Technologies
A key reason this therapy is so exciting isn’t just that it works—it’s that it’s built on technologies already shown to be safe in humans. mRNA vaccines, lipid nanoparticles, and CAR T cells are no longer exotic or theoretical. Hundreds of thousands of patients have received them, and their safety profiles are well understood.
“We’re not starting from scratch,” Kwong emphasizes. “That’s what makes the road to clinical translation so promising. The building blocks are already in place.”
Lipid nanoparticles have been used to deliver billions of mRNA vaccine doses globally with minimal side effects. Similarly, CAR T cell therapies for blood cancers are FDA-approved and have been administered in hospitals for nearly a decade.
By combining these two modalities into a unified treatment strategy, the Georgia Tech team has created a hybrid therapy that could be tested in humans much sooner than other experimental approaches.
Why Local Control Matters
Another core innovation of the therapy is its focus on local disease control—catching cancer early and stopping it before it spreads. Kwong argues that too much attention is focused on treating advanced, metastasized cancers, and not enough on preventing that metastasis in the first place.
“Most terminal cancer cases are the result of local disease spreading. If we could control tumors when they’re still localized, we could dramatically reduce mortality,” he says.
This therapy could be injected directly into a solid tumor, such as one in the breast or colon, flag it for destruction, and possibly train the immune system to patrol for any future outbreaks. That would represent a monumental shift in cancer management—from fighting a fire to fireproofing the house.
The Path Ahead: From Lab Bench to Hospital Bed
Despite its promise, this therapy still has hurdles to clear before reaching the clinic. Animal models, though informative, aren’t perfect analogs for human cancer. More preclinical testing is needed to confirm safety, dosing, delivery, and long-term effects.
But the design of the therapy was engineered with the clinic in mind from the beginning.
“We deliberately chose components that regulators and doctors are already familiar with,” Kwong notes. “That streamlines the path toward human trials.”
One of the next steps will involve optimizing the lipid nanoparticle formulation to ensure precise delivery to tumor cells with minimal leakage into healthy tissue. The researchers are also developing strategies for producing the engineered CAR T cells more efficiently and cost-effectively—a major consideration for any personalized medicine approach.
And, crucially, they’re exploring how this strategy could be adapted to treat metastatic disease, where tumors are not localized and can’t be directly injected. One possibility is engineering nanoparticles that home in on tumor environments systemically—essentially letting the synthetic antigen hunt down the cancer wherever it hides.
A New Era of Immunotherapy?
If successful, this platform could change not just how we treat cancer—but how we think about it.
The immune system is already our most powerful defense mechanism. For decades, researchers have tried to coax it into recognizing cancer the same way it recognizes viruses or bacteria. But without the right “ID tag,” even the most advanced immune cells can overlook tumors hiding in plain sight.
Georgia Tech’s solution is elegantly simple: give the cancer an ID tag the immune system can’t miss, then train the immune cells to do what they do best—defend the body.
The idea seems almost obvious in retrospect. But it took years of work, cross-disciplinary collaboration, and an inventive leap into synthetic biology to make it a reality.
The potential rewards? Life-saving treatments for millions of patients with hard-to-treat cancers. Long-term immunity against recurrence. And perhaps a future where cancer is no longer feared as an invisible killer—but seen, targeted, and destroyed before it ever takes hold.
Reference: Lena Gamboa et al, Sensitizing solid tumors to CAR-mediated cytotoxicity by lipid nanoparticle delivery of synthetic antigens, Nature Cancer (2025). DOI: 10.1038/s43018-025-00968-5