Glioblastoma is not the kind of enemy that shouts its arrival. It does not announce itself with grand tumors in the lungs or liver or bones. Instead, it seeps into the brain like a ghost, silently slipping between cells, embedding itself into places no surgeon can safely reach. For patients and physicians alike, it is a diagnosis heavy with dread.
Among all primary brain cancers, glioblastoma is the most common and the deadliest. Its survival statistics have barely budged in decades. Standard therapies—surgery, radiation, and chemotherapy—often feel like fighting a wildfire with a garden hose. The tumor may shrink temporarily, but the embers it leaves behind almost always ignite again.
What makes glioblastoma so lethal is not just its aggressiveness but the way it spreads. It doesn’t travel to distant parts of the body, like many other cancers. Instead, it infiltrates the brain locally, crawling along white matter, weaving through the cortex, and vanishing into the shadows between neurons. Finding a way to block that silent invasion has long been one of neuro-oncology’s greatest unmet challenges.
But now, an international team of scientists may have taken a significant step toward cracking the code of how glioblastoma spreads—and more importantly, how it might be stopped.
Following the Tracks
Led by researchers at Uppsala University in Sweden, in collaboration with Queen Mary University of London and the Dana-Farber Cancer Institute in Boston, the team recently published groundbreaking findings in Nature Communications that shed light on the microscopic strategies glioblastoma cells use to navigate the brain.
What they discovered may seem surprisingly logical: not all glioblastoma cells spread in the same way. Some follow blood vessels like rails, hugging the vasculature as if it were a map. Others disperse more randomly, meandering through the brain tissue with a patternless determination. But what drives a cell to choose one route over another?
The answer, as it turns out, lies in the cell’s internal identity—its “state,” dictated by the specific genes it activates.
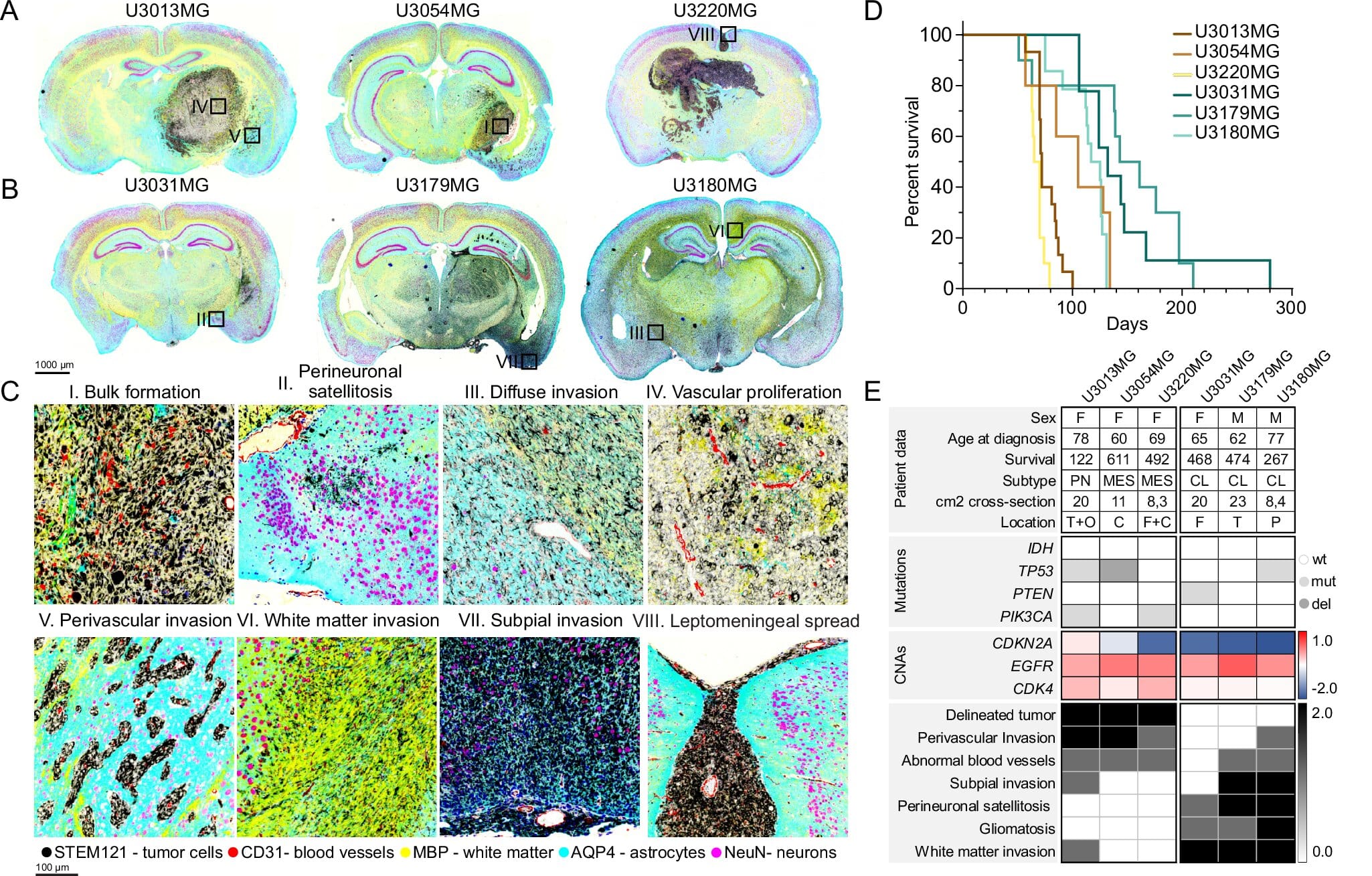
Using state-of-the-art single-cell analysis and spatial protein profiling techniques, the researchers mapped both gene activity and physical location in tumor samples from mice and humans. It allowed them to peer into the tumor like never before—seeing not only what each individual cell was doing, but also where it was going.
What they found was a cellular orchestra with multiple players. And each player had a different role in invasion.
The Architects of Invasion
Three genes emerged as key players in determining how glioblastoma cells spread. The first, ANXA1, was strongly linked to cells that invade along blood vessels. These cells don’t wander aimlessly; they travel purposefully, as if marching along highways that supply nutrients and oxygen.
The other two genes, HOPX and RFX4, were tied to a very different type of spread—diffuse infiltration. These cells do not follow roads. They sneak through the underbrush, dissolving into the brain’s dense neural networks and becoming nearly impossible to detect until it’s too late.
To test whether these genes were just markers of invasion or actual drivers, the team used gene editing to knock them out in mouse models. When ANXA1 was disabled, the tumor cells no longer clung to blood vessels. Similarly, removing HOPX or RFX4 disrupted the diffuse infiltration pattern.
More than that, in many cases, the animals survived longer.
That shift in behavior was more than encouraging. It showed that the invasion patterns weren’t just a result of random mutation or environmental chance—they were, in part, hard-coded into the tumor’s genetic instructions. And if those instructions could be rewritten, the behavior of the tumor could be altered.
A Patchwork Tumor
One of the most important insights from this research is that glioblastoma is not a uniform enemy. It’s not one disease—it’s many.
Within the same tumor, cells can exist in completely different states. Some are aggressive travelers, others are rooted builders. Some spread with stealth; others blaze forward with speed. This diversity is what makes glioblastoma so hard to treat. A drug that works on one cell type may do nothing to another just millimeters away.
But by understanding these cell states and how they correlate with invasion patterns, researchers can begin to imagine smarter treatments—ones that don’t just target the tumor, but the paths it uses to grow.
Professor Sven Nelander, the senior author of the study, sees this as a turning point. “Our findings show that glioblastoma is not a homogenous disease but is made up of several cell types with different invasion patterns,” he explained. “Understanding and controlling these patterns opens up new opportunities for targeted treatments.”
The key is not just to stop the tumor, but to reroute it, slow it down, and make it vulnerable.
Markers of Fate
In addition to understanding how these genes influence behavior, the research also uncovered something deeply valuable: biomarkers.
When the researchers examined human tumor tissue, they found the proteins made by ANXA1 and RFX4 present in many samples. Even more striking, the presence of these proteins was associated with poorer survival outcomes in patients. That makes them more than just indicators of invasion style—they may be predictors of prognosis.
In clinical terms, this could be a game changer. If doctors can test a tumor for ANXA1 or RFX4 levels early on, they might be able to estimate how aggressive the disease will be and tailor treatment accordingly.
And down the road, those proteins themselves could become therapeutic targets. Blocking them might not cure glioblastoma outright, but it might limit its ability to infiltrate the brain—turning a deadly sprint into a manageable crawl.
Hope Through Complexity
Cancer research is rarely a straight line. Each breakthrough is often followed by more questions. Why do some tumors express these genes while others don’t? What switches them on or off? How do treatments like chemotherapy or immunotherapy affect these invasion states?
And yet, in the face of that complexity, this study offers a profound sense of hope. Not because it has all the answers—but because it proves the tumor isn’t acting entirely at random. It has logic. It has rules. And what has rules can, at least in theory, be rewritten.
For patients, families, and clinicians facing glioblastoma, this discovery is a reminder that progress is still possible. That even in the darkest corners of the brain, light can reach.
Looking Forward
The fight against glioblastoma remains one of modern medicine’s greatest challenges. It is a disease that resists, adapts, and endures. But with research like this, the wall of mystery is beginning to crack.
By understanding how glioblastoma cells choose their routes, and how those choices are governed by identifiable genetic states, scientists are moving closer to treatments that are not just reactive, but strategic.
Imagine a future where we don’t just attack the tumor—we trap it, redirect it, confuse it, slow it. A future where a diagnosis of glioblastoma doesn’t feel like an ending, but the beginning of a plan.
This research is not the final word. But it’s an extraordinary sentence in a growing story—a story where, for the first time in a long time, glioblastoma’s secrets are being told.
Reference: Milena Doroszko et al, The invasion phenotypes of glioblastoma depend on plastic and reprogrammable cell states, Nature Communications (2025). DOI: 10.1038/s41467-025-61999-1