Every time you open a fizzy bottle and hear that familiar hiss, a quiet transformation begins. The cool sweetness of a soft drink may seem like a simple pleasure, a sugary treat that briefly lifts your spirits. But inside your gut—far beyond the reach of taste and intention—this sugar rush awakens a microscopic drama. The characters? Millions of gut bacteria that call your intestines home. And the twist? They’re rewriting their own genetic code in response to your diet.
This isn’t science fiction. It’s the emerging reality of microbiome research, and it’s unveiling how our choices—especially what we eat and drink—shape our biology in real time. According to groundbreaking new research published in Nature Communications, the white sugar in soft drinks doesn’t just give you a blood sugar spike. It causes your gut bacteria to flip pieces of their DNA—tiny but powerful molecular switches—altering how they behave, how they interact with your immune system, and potentially, how your body reacts to the world around it.
The silver lining? The damage isn’t permanent. But the implications are deep.
The Bacterial Orchestra Within
Your gut is home to trillions of bacteria, fungi, and other microorganisms—collectively known as the microbiome. These microscopic allies have evolved alongside humans for millennia, forming an intricate symbiosis. They don’t just help digest food. They produce vitamins, defend against pathogens, and help train the immune system to distinguish between friend and foe.
One of the most essential species in this community is Bacteroides thetaiotaomicron—a mouthful of a name for a bacterium that performs heroic roles. It helps maintain the mucus layer that protects your intestinal walls, wards off harmful microbes, and even communicates with your immune cells.
But like any living being, these bacteria are influenced by their environment. And in the gut, that environment changes constantly—with every meal, every medication, every emotional upheaval. To survive, these microbes must remain flexible. This ability to shift and adapt is known as functional plasticity.
Tiny Flips with Big Consequences
Prof. Naama Geva-Zatorsky and her team at the Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, have been studying how gut bacteria adapt so quickly. In earlier work, they discovered a fascinating mechanism: DNA inversions. Think of it as a molecular light switch. Certain segments of a bacterium’s DNA can flip orientation—turning genes on or off, much like flipping a sentence backward in a book. This process enables the bacteria to rapidly adjust how they function without changing their core genetic material.
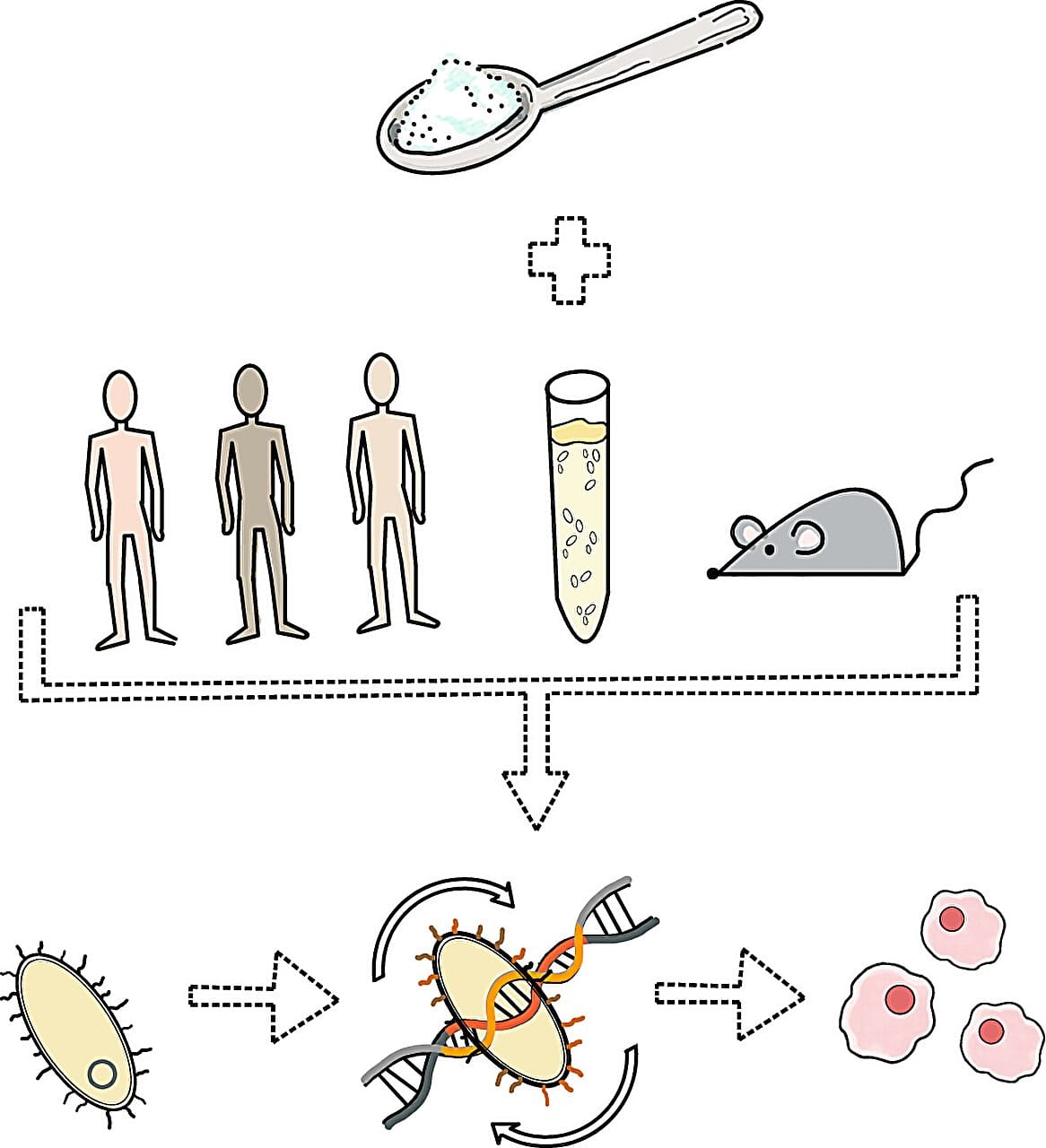
In their latest study, led by Geva-Zatorsky and Ph.D. student Noa Gal-Mandelbaum, the team explored what happens to these DNA switches when mice, and even humans, consume common dietary components—especially white sugar found in soft drinks.
Using advanced proteomics and molecular biology tools, with the help of Dr. Tamar Ziv and the Smoler Proteomics Center, the researchers tracked changes in the inversion profiles of Bacteroides thetaiotaomicron. What they found was startling: white sugar consumption prompted these bacteria to flip their DNA switches—altering the way they behaved inside the gut.
From Sugar to Immune Storm
When the bacteria flipped their DNA, their interactions with the host’s immune system changed significantly. The researchers observed shifts in several immune markers. T-cells—crucial white blood cells that protect the body against infections and cancers—were affected. Cytokines, the signaling molecules of the immune system, began sending different messages. Even the gut’s permeability changed, meaning the intestinal wall became more or less “leaky” depending on the microbial shifts.
This is no small matter. A leaky gut can allow toxins, partially digested food, and microbes to pass into the bloodstream—triggering systemic inflammation and potentially contributing to autoimmune disorders, allergies, and other chronic conditions.
In short, sugar wasn’t just feeding bacteria—it was reshaping them. And in doing so, it was tugging on the strings of the immune system.
The Body’s Resilience
But the story doesn’t end in disaster. When the researchers removed white sugar from the mice’s diet, something remarkable happened: the bacterial DNA began to revert. The inversion patterns that had changed flipped back. The immune markers normalized. It was as if the gut, shaken by the storm, found its way back to still waters.
This reversibility offers hope. It means that our gut ecosystem, though sensitive, is not fragile. It can adapt, repair, and reset. But it also means that our daily choices matter profoundly—not just over years, but days, even hours.
Soft drinks and added sugars don’t just leave a temporary mark on our metabolism. They ripple into the microscopic symphony within us, altering the notes and rhythms of how our body functions.
Toward Personalized Nutrition
This study marks more than a scientific breakthrough—it signals a shift in how we might approach nutrition, health, and disease in the future. For decades, dietary advice has focused on broad strokes: eat less sugar, eat more fiber, avoid saturated fat. But the emerging science of the microbiome suggests a far more nuanced reality.
Geva-Zatorsky’s team believes that by understanding how individual nutrients alter microbial genetics, we may one day craft personalized dietary recommendations—not just to prevent disease, but to actively fine-tune the immune system. Imagine a future where your doctor can analyze your gut bacteria and give you a tailored eating plan to reduce inflammation, boost immunity, or manage autoimmune flare-ups.
Food would no longer be just fuel or pleasure—it would be a dialogue with your own biology.
The Responsibility in Every Bite
Perhaps the most profound takeaway from this research isn’t the complexity of microbial genetics, but the simplicity of its message: what we consume reshapes us. Not just metaphorically, but biologically. Not slowly, but immediately.
When you drink a can of soda, you’re not just satisfying a sweet tooth. You’re sending a message to your microbiome. You’re pulling the strings on the DNA of creatures living inside you, and through them, you’re nudging your own immune system in directions science is only beginning to understand.
And yet, this knowledge is empowering. Because it means we’re not passive passengers in our health journey. We are active participants, with agency over how our body evolves in response to the environment we create for it.
The Gut Knows
As research into the microbiome accelerates, it’s becoming increasingly clear that the gut is not just a digestive organ—it’s a decision-making center. It hosts a network of microbes with the capacity to adapt, influence mood, shape immunity, and respond to the foods we eat with astonishing precision.
Soft drinks and white sugar are not inherently evil. But their effects run deeper than we once imagined. They don’t just rot teeth or spike insulin. They trigger molecular decisions that ripple through the gut and into the bloodstream. They flip switches in the bacterial genome, prompting our microbial partners to change course—and, sometimes, to alter how our own cells behave.
But the body forgives. When sugar is removed, the switches can flip back. The bacteria recalibrate. The immune system finds equilibrium again. It’s a hopeful reminder that healing is possible, that change is reversible, and that every sip, every bite, is a choice with power.
The next time you hold a soft drink in your hand, pause. Think of the billions of lives inside you waiting to respond. Their fate—and perhaps your own—might just depend on what you choose next.
Reference: Noa Gal-Mandelbaum et al, Dietary carbohydrates alter immune-modulatory functionalities and DNA inversions in Bacteroides thetaiotaomicron, Nature Communications (2025). DOI: 10.1038/s41467-025-60202-9