For years, scientists have known that not all fat in the body behaves the same way. Some fat seems eager to melt away with diet and exercise. Other fat clings stubbornly, resisting change. But deep inside our skeletons lies something even stranger.
At WashU Medicine, a team of researchers began asking a simple but unsettling question: Why does a large portion of our body fat refuse to budge at all?
The answer, they discovered, begins not in the belly or thighs, but in the brain.
Their findings, published in Nature Metabolism, describe a surprisingly powerful biological pathway—one that can erase nearly all body fat without reducing how much food is eaten. It is a discovery as astonishing as it is cautionary.
The Fat That Would Not Listen
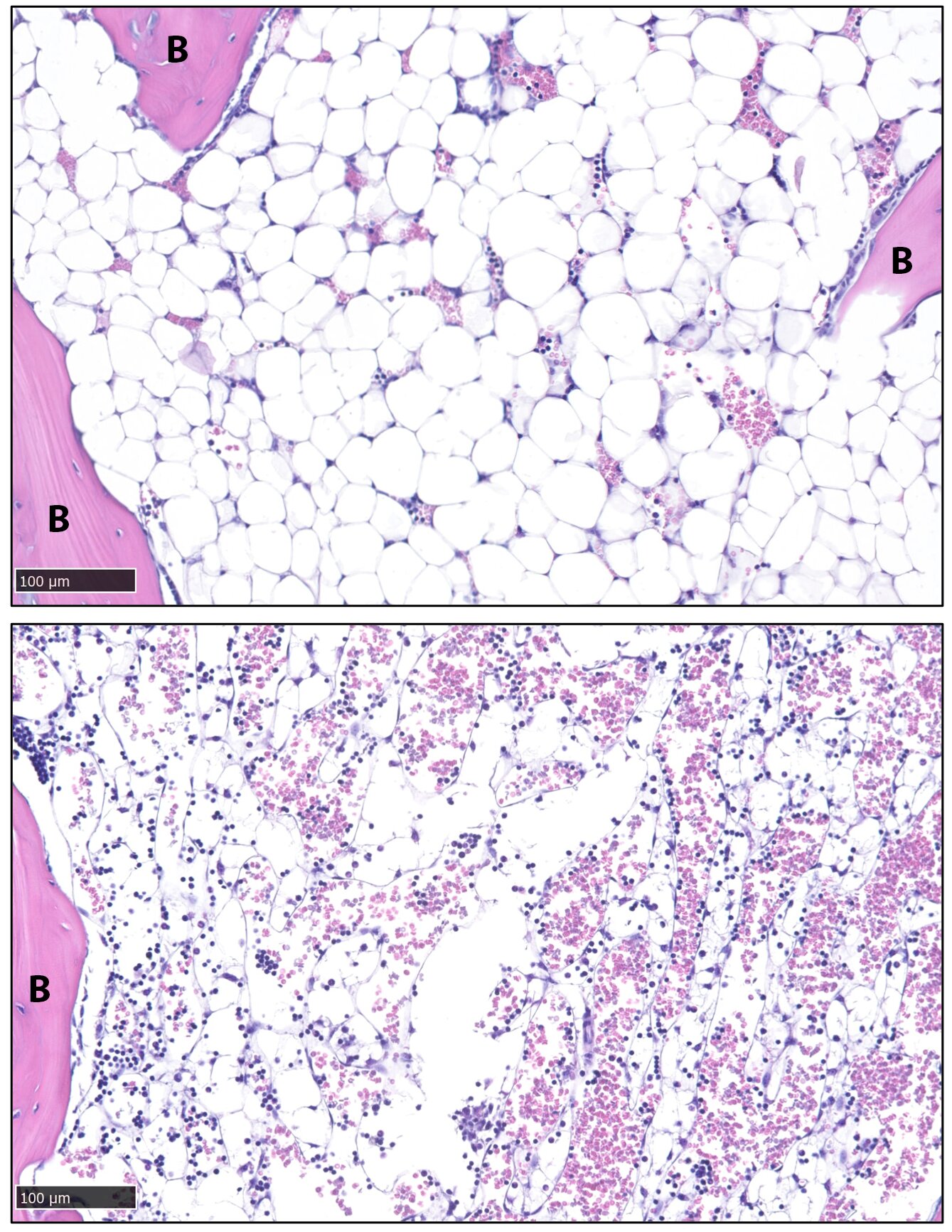
About 70% of bone marrow is filled with fat. This isn’t the kind of fat people talk about losing before summer. It sits deep within the skeleton, tucked inside bones, quietly occupying space that many assume belongs only to blood cells and structural tissue.
But this marrow fat behaves differently from ordinary fat.
“It doesn’t respond to diet or exercise,” explained senior author Erica L. Scheller, DDS, Ph.D., an associate professor in the Division of Bone and Mineral Diseases. No matter how the body’s energy balance shifts, this fat remains.
The research team, including Xiao Zhang, Ph.D., and graduate student Sree Panicker, focused on a special group of fat cells called constitutive bone marrow adipocytes. These cells, embedded deep in the skeleton, seemed almost immune to change.
Zhang gave them another name: stable adipocytes.
Stable, because they resist the usual biological signals that tell fat cells to break down and release energy.
The question was no longer just why they exist—but how they remain so immovable.
The Molecular Lock That Protects Fat
As the researchers peered closer at these cells, they uncovered a crucial clue. Stable adipocytes expressed unusually high levels of proteins that inhibit fat breakdown.
In simple terms, these cells are biologically wired to defend themselves.
Normally, when the body needs energy, fat cells undergo a process that breaks stored fat into usable fuel. But these stable adipocytes carry strong internal brakes. Those inhibitory proteins keep fat locked safely inside.
This explains why diet and exercise fail to affect them. The molecular machinery required for fat breakdown is present—but restrained.
It was as if these cells were sealed vaults.
But what could open them?
A Signal From the Brain
The breakthrough came from an unexpected direction. The researchers explored the effects of leptin, a hormone known to act in the brain.
In experiments with mice, they delivered sustained injections of leptin directly into the brain. What happened next startled them.
The brain responded by shifting the body into a state of low glucose and low insulin. These metabolic changes rippled throughout the body. And in that altered state, something extraordinary occurred.
The inhibitors inside the stable adipocytes diminished.
The molecular brakes loosened.
Within days, the mice experienced a complete loss of body fat—even though they continued eating normally.
The animals did not reduce food intake. They were not starving. Yet their fat stores vanished.
This pathway, beginning in the brain and traveling through metabolic signals, proved capable of unlocking even the most resistant fat cells in the body.
It was a biological override switch.
Power That Demands Caution
The implications are both exciting and alarming.
Stable adipocytes are not limited to bone marrow. They are found in the hands, feet, and around important glands. In certain severe wasting disorders, when fat within these cells is lost, patients can suffer from bone fractures and a diminished quality of life.
Fat, often viewed as an enemy, clearly serves protective roles in specific tissues. Removing it indiscriminately could have serious consequences.
The researchers emphasize that this pathway is so potent that it should not be applied to humans until it is much better understood. Triggering widespread fat loss may sound desirable in an age focused on obesity, but fat inside bone and around critical structures plays structural and physiological roles.
In severe wasting conditions, the loss of stable fat is not a victory—it is a warning sign.
The discovery, therefore, carries a dual edge.
Two Very Different Futures
On one hand, understanding how stable adipocytes resist breakdown could help protect vulnerable patients. If scientists can define the mechanisms behind stable fat loss, they may be able to prevent harmful fat depletion in those suffering from wasting disorders.
Preserving fat in bone marrow could mean protecting bones from fractures. It could mean maintaining strength, stability, and quality of life.
On the other hand, learning how to safely activate fat loss in stubborn adipocytes might one day contribute to treatments for obesity. Many people struggle with fat that seems biologically resistant to change. Understanding this brain-driven pathway could open new therapeutic possibilities.
But the researchers tread carefully. The pathway is powerful, and power in biology must be handled with respect.
Why This Discovery Matters
This research changes how we think about fat.
It reveals that some fat cells are not passive storage depots but highly regulated, biologically protected structures. It shows that the brain can orchestrate profound changes in body composition without altering food intake. And it highlights the delicate balance between health and harm.
Most importantly, it reminds us that the body is not divided into simple categories of good and bad. Fat can protect bones. Hormones can unlock hidden cellular defenses. The brain can command sweeping metabolic change.
By uncovering the pathway linking the brain, leptin, low glucose and insulin, and the breakdown of even the most stable adipocytes, scientists have illuminated a hidden layer of metabolic control.
The discovery does not offer a quick fix. It offers understanding.
And in science, understanding is the first step toward healing—whether that means preserving fragile fat in wasting disorders or carefully targeting stubborn fat in obesity.
Deep inside our bones, silent fat cells have been keeping their secrets for decades. Now, at last, we are beginning to listen.
Study Details
Xiao Zhang et al, A catecholamine-independent pathway controlling adaptive adipocyte lipolysis, Nature Metabolism (2026). DOI: 10.1038/s42255-025-01424-5