Inside every cell of the human body lies a quiet drama, one that unfolds trillions of times over a lifetime. At its center are mitochondria, often described as the “powerhouses of the cell.” These organelles generate the energy that fuels life, but they are far more than miniature batteries. Mitochondria communicate with the rest of the cell, respond to stress, and influence survival. They are both guardians and potential saboteurs of health.
A recent study from the Max Planck Institute for Biology of Aging in Cologne has revealed an unexpected twist in this mitochondrial story. The researchers showed that when the delicate balance of genetic building blocks goes awry, ribonucleotides—normally destined for RNA—slip into mitochondrial DNA (mtDNA). This tiny molecular misstep sets off a cascade of immune responses that link directly to inflammation, aging, and disease.
The findings, published in Nature under the title “Ribonucleotide incorporation into mitochondrial DNA drives inflammation,” shine a spotlight on how molecular mistakes ripple outward, connecting the integrity of mtDNA to some of the deepest questions in biology: Why do we age? Why do tissues fail? And can we intervene?
Mitochondria as Keepers of Balance
Mitochondria sit at the crossroads of metabolism, signaling, and survival. They produce ATP, the cell’s universal energy currency, but they also decide when a cell should live or die. In health, mitochondria are a source of vitality; in dysfunction, they become harbingers of inflammation, cell death, and chronic disease.
Their genomes, small circular strands of DNA inherited maternally, are particularly vulnerable. Unlike nuclear DNA, mtDNA lacks robust repair systems, and its proximity to reactive oxygen species generated during respiration makes it fragile. Maintaining mtDNA integrity is essential, but it is also precarious.
The new research highlights an underappreciated threat: an imbalance between ribonucleotides (rNTPs) and deoxyribonucleotides (dNTPs), the molecular building blocks of RNA and DNA. Under normal circumstances, DNA polymerases use dNTPs exclusively to replicate DNA. But if rNTPs become too abundant relative to dNTPs, errors occur. Ribonucleotides sneak into DNA, producing a genome laced with mistakes that mitochondria cannot easily repair.
When Missteps Become Signals
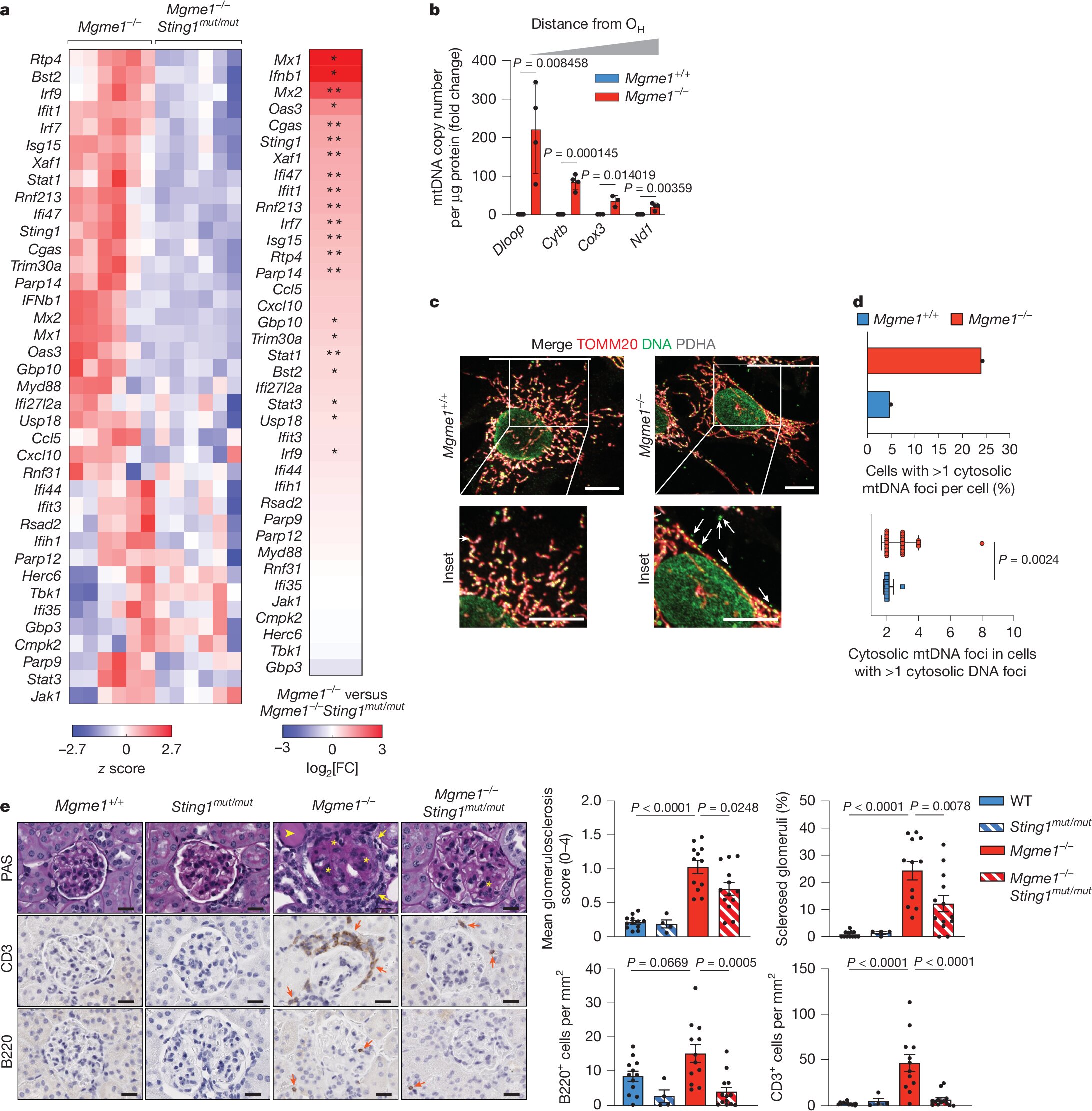
In the study, the scientists focused on MGME1, an enzyme that helps maintain mtDNA fidelity. Mice lacking MGME1 displayed higher ratios of rNTPs to dNTPs in their mitochondria. This imbalance led to ribonucleotide misincorporation into mtDNA, creating molecular damage that did not remain silent. Instead, it triggered an immune alarm.
The culprit was the cGAS–STING pathway, part of the innate immune system. Normally, this pathway detects viral or bacterial DNA in the cytoplasm and mounts a defensive response. But when damaged mtDNA accumulates or leaks into the wrong compartments, the immune system mistakes it for foreign invaders. cGAS senses the aberrant DNA, STING transmits the signal, and TBK1 amplifies it—leading to inflammation.
This pathway is vital for short-term defense against infection, but when chronically activated, it pushes cells into a prolonged state of alarm. The result is not resilience but decline. The study demonstrated that mtDNA errors due to nucleotide imbalance are sufficient to provoke this chain reaction, linking metabolic missteps to immune overdrive.
Senescence and the Whisper of Aging
One of the most striking observations from the research was the connection to cellular senescence. Senescent cells are those that stop dividing but refuse to die. Instead, they adopt a secretory phenotype, releasing inflammatory molecules that alter the tissue environment around them. This so-called “senescence-associated secretory phenotype” (SASP) is increasingly recognized as a driver of aging and age-related disease.
The researchers showed that senescent human fibroblasts exhibited elevated rNTP to dNTP ratios, much like MGME1-deficient mouse cells. Tissues from aged mice—including the liver, kidney, spleen, and heart—also displayed this imbalance. The conclusion was clear: as organisms age, the equilibrium of nucleotides shifts, leaving mtDNA vulnerable. That vulnerability fuels cGAS–STING-driven inflammation, tying together the threads of metabolism, immunity, and senescence.
It is as if the mitochondria, once faithful keepers of energy, begin to whisper signals of distress. Those whispers accumulate, spreading inflammation through tissues, and ultimately contribute to the decline we call aging.
Tipping the Balance
The study did not stop at identifying the problem. It asked: what happens if the nucleotide balance is restored?
In MGME1-deficient cells, lowering SAMHD1—an enzyme that reduces dNTP pools—worsened inflammation. Conversely, supplementing senescent cells with deoxyribonucleosides, which boost dNTP levels, reduced inflammatory gene expression linked to the cGAS–STING pathway.
These experiments offered proof of principle: altering the balance of rNTPs and dNTPs changes the inflammatory outcome. Mitochondrial genome instability is not an unchangeable fate. Interventions at the level of nucleotide metabolism can influence immune signaling, hinting at therapeutic opportunities.
From Mice to Medicine
The implications of these findings reach far beyond the laboratory. Chronic activation of the cGAS–STING pathway has been implicated in autoimmune disorders, neurodegeneration, kidney disease, and cancer. If ribonucleotide incorporation into mtDNA is one of the sparks that ignites this inflammatory cascade, then correcting nucleotide imbalances could represent a new frontier in medicine.
Imagine therapies that fine-tune the pools of nucleotides in aging cells, keeping mitochondria functional and their genomes intact. Such treatments might quiet the inflammatory chatter of senescent cells, preserving tissue health for longer. They could reduce vulnerability to neurodegenerative diseases where inflammation and mitochondrial dysfunction intertwine, or slow the progression of kidney disease and cancer where immune activation runs rampant.
It is, of course, an early vision. The leap from mice and cell cultures to human therapies is vast. Yet the conceptual breakthrough is profound: inflammation in aging may not simply be a passive consequence of wear and tear, but an active response to specific molecular imbalances that we might correct.
A Hidden Vulnerability
One haunting detail in the study is that mtDNA lacks any known mechanism to correct ribonucleotide misincorporation. Nuclear DNA has multiple repair pathways, but mitochondria seem defenseless. Whether such a repair system once existed and was lost, or whether it never evolved, is unclear. For now, mitochondria rely on the balance of nucleotides to prevent errors in the first place. When that balance tips, they fall silent victims.
If nature has not given mitochondria a tool to handle this imbalance, perhaps science can. Future research may discover ways to “take out the mitochondrial trash,” repairing or replacing faulty mtDNA, or adjusting nucleotide pools to maintain fidelity. Such interventions could extend not only the health of mitochondria but the vitality of entire tissues and organisms.
The Larger Story of Aging
Aging is often seen as inevitable, an unstoppable slide into decline. But biology increasingly paints a more nuanced picture. Aging is not a single process but an accumulation of many small failures—DNA damage, protein misfolding, stem cell exhaustion, immune dysregulation. Each failure creates ripples that spread through tissues and organs.
The work from Cologne places nucleotide imbalance and mtDNA integrity at the heart of this tapestry. It shows how the smallest molecular missteps can awaken ancient immune pathways, transforming cells from quiet workers into sources of chronic inflammation.
This is not a tale of doom but of opportunity. By uncovering these hidden vulnerabilities, we learn where interventions might matter most. Aging may never be erased, but it may be softened, delayed, or reshaped.
Toward a Future of Resilience
The story of ribonucleotides in mitochondrial DNA is a story of connection. It connects metabolism to immunity, DNA chemistry to inflammation, and molecular mistakes to the lived reality of aging tissues. It reminds us that biology is not just mechanics but narrative—an interplay of actors, signals, and consequences that span scales from the atomic to the organismal.
And it points toward a future where interventions could prolong resilience. If we learn to protect mtDNA from ribonucleotide imbalance, if we find ways to silence unnecessary cGAS–STING alarms, we may extend not only lifespan but healthspan—the years of life lived free from chronic disease.
The mitochondria, once thought of only as cellular batteries, emerge here as storytellers of aging. They tell us that balance matters, that small errors can reverberate across decades, and that science has the power to rewrite some of the ending.
The study’s findings may one day be remembered as more than an academic discovery. They could be the first step toward therapies that let us live longer, healthier lives by safeguarding the integrity of the very organelles that power us.
More information: Amir Bahat et al, Ribonucleotide incorporation into mitochondrial DNA drives inflammation, Nature (2025). DOI: 10.1038/s41586-025-09541-7