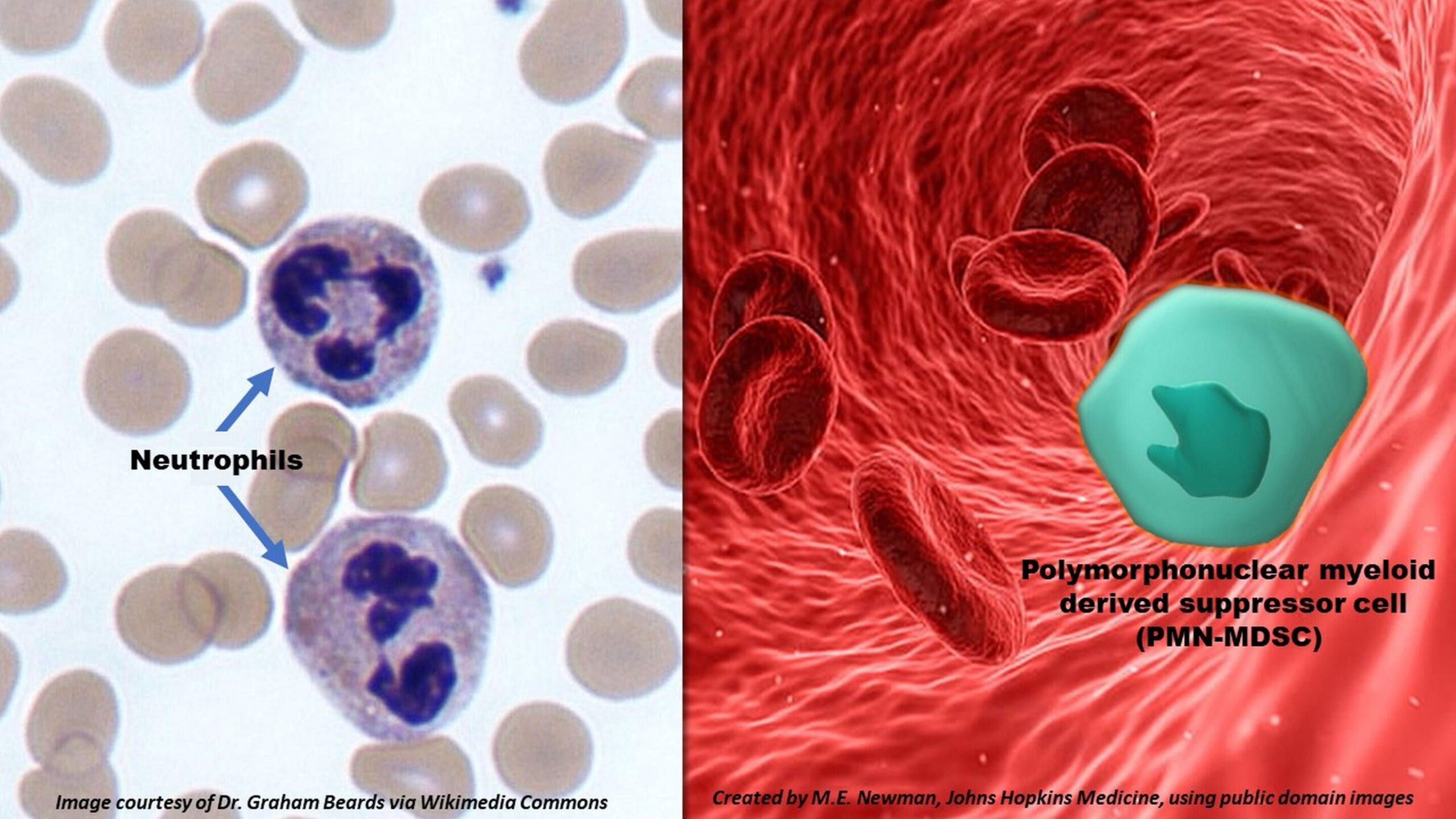
In the intricate battlefield of our immune system, few warriors are more trusted or relied upon than neutrophils. These microscopic first responders swarm to the site of infection within minutes, engulfing pathogens, releasing deadly enzymes, and rallying other immune cells into action. But what happens when the soldiers stop fighting for the body—and start working against it?
A startling new study by researchers at Johns Hopkins Medicine, the Bloomberg School of Public Health, and the Whiting School of Engineering has uncovered an alarming twist in the story of COVID-19. According to findings published in Science Translational Medicine, the virus behind COVID-19—SARS-CoV-2—appears capable of reprogramming neutrophils into a form that doesn’t just stand down but actively suppresses the immune system’s most powerful virus-fighting forces.
A Trojan Horse Within
At the heart of this discovery lies a rare cellular transformation. In patients with severe COVID-19, researchers found that neutrophils no longer behaved like aggressive pathogen killers. Instead, they morphed into polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs), a type of immune cell typically associated with cancer and chronic inflammation—not viral infections.
“This reprogramming essentially turns neutrophils from immune warriors into saboteurs,” explains Dr. Andrea Cox, senior author of the study and a professor of medicine and oncology at Johns Hopkins. “Rather than helping the body fight off SARS-CoV-2, they suppress T cells, the very agents responsible for clearing viruses.”
This reprogramming isn’t just a biological curiosity—it may offer critical insight into why certain cases of COVID-19 become so dangerous, even life-threatening.
From Emergency Rooms to the Lab
The study examined blood samples from 39 patients hospitalized with severe COVID-19 at Johns Hopkins hospitals in 2020, before the availability of vaccines. For comparison, samples from nine healthy individuals served as controls. All participants were unvaccinated, and importantly, none of the COVID-19 patients had received immune-suppressing corticosteroids like dexamethasone—ensuring that the immune cell behavior observed was driven by the virus, not medical intervention.
“We were struck by what we saw,” says Dr. Leon Hsieh, lead author and former doctoral student in Cox’s lab. “The neutrophils in COVID-19 patients had degranulated—meaning they had expelled their internal arsenals—and were showing characteristics we’ve previously only seen in cancer or other nonviral illnesses.”
These neutrophils weren’t just losing their functionality—they were mutating into something dangerous.
A New Player in Viral Pathogenesis: PMN-MDSCs
The transformation into PMN-MDSCs is no minor switch. These cells are infamous in cancer biology for their ability to suppress T cells, which play a pivotal role in attacking tumors and fighting infections. PMN-MDSCs achieve this suppression through various mechanisms, one of which is the expression of immune checkpoint proteins like PD-L1.
PD-L1, or programmed death ligand-1, binds to PD-1 receptors on T cells. This binding acts like a biological “brake,” preventing T cells from activating, multiplying, or sending out distress signals via cytokines. It’s a mechanism that tumors hijack to evade immune surveillance—and now, SARS-CoV-2 seems to be employing a similar tactic.
“When we compared neutrophils from patients with mild or moderate COVID-19 to those with severe disease, the severe cases had neutrophils that expressed significantly more PD-L1 and another protein, LOX-1,” Hsieh explains.
While LOX-1’s role in this transformation is still not fully understood, PD-L1’s effects are well documented: it directly undermines the immune response.
Direct Manipulation by the Virus
To test whether the virus itself could drive this transformation, researchers cultured healthy neutrophils in the presence of SARS-CoV-2 in the lab. The results were remarkable: exposure to the virus was enough to trigger the conversion of neutrophils into PMN-MDSCs, complete with the ability to suppress T cell proliferation and inhibit cytokine production.
Even more intriguingly, when the researchers repeated the experiment using the H1N1 influenza virus—a respiratory virus known for causing severe illness—the transformation didn’t occur.
“This strongly suggests that SARS-CoV-2 has a unique capacity to rewire neutrophils in a way other respiratory viruses cannot,” Hsieh says. “It’s not just a matter of overwhelming the immune system—it’s an active subversion.”
A New Therapeutic Frontier?
With this mechanistic insight comes a tantalizing possibility: could this rogue neutrophil behavior be stopped?
The researchers turned to an established weapon in the cancer immunotherapy arsenal—antibodies against PD-L1. In laboratory experiments, introducing PD-L1-blocking antibodies into cultures of virus-exposed neutrophils significantly reduced their ability to suppress T cells. T cells began producing cytokines again, signaling a reawakening of the immune response.
“These findings suggest that PD-L1 blockade, already used in cancer treatment, could be repurposed to restore immune function in severe COVID-19,” says Cox. “It’s a strategy that could complement antiviral drugs or provide a fallback for patients who can’t tolerate such medications.”
However, she cautions that these results are preliminary. Clinical trials would be necessary to evaluate both safety and efficacy in COVID-19 patients. But in a landscape still riddled with viral variants and long-term complications, the prospect of a new therapeutic tool is a ray of hope.
The Bigger Immunological Picture
This discovery adds another layer of complexity to our understanding of COVID-19, a disease that continues to defy simple explanations. For much of the pandemic, attention has focused on the virus’s spike protein, mutations that affect transmission, or the inflammatory “cytokine storm” seen in critically ill patients. But this study shifts the focus back to a more fundamental question: what happens to our immune system’s architecture when this virus invades?
The answer, it seems, is both startling and subtle. SARS-CoV-2 doesn’t just provoke a massive immune response—it manipulates its key players, turning them against their allies.
It’s an elegant, if sinister, evolutionary adaptation. By transforming neutrophils—the most abundant white blood cells in our body—into suppressors of the immune system, the virus avoids complete destruction and carves a path to long-term illness or death in vulnerable hosts.
The Road Ahead
The implications of this study go far beyond COVID-19. They hint at a broader biological principle: that immune cells, even ones as essential and seemingly straightforward as neutrophils, are remarkably plastic. Under certain conditions, they can be reprogrammed to do the opposite of their intended function.
Understanding the triggers and consequences of this transformation opens new doors for immunology, virology, and therapeutic development. Could other chronic viral infections, like HIV or hepatitis C, also exploit similar pathways? Could early intervention to block neutrophil reprogramming reduce the risk of long COVID?
“These are the kinds of questions we’re excited to explore next,” says Cox. “COVID-19 has been a tragedy, but it has also accelerated our understanding of immune dynamics in ways we never expected.”
As science continues to peel back the layers of this viral foe, one lesson becomes increasingly clear: the immune system, once seen as a blunt force of nature, is in fact a nuanced and programmable network—one that pathogens, and perhaps soon, doctors, can rewire for better or worse.
Reference: Leon L. Hsieh et al, SARS-CoV-2 induces neutrophil degranulation and differentiation into myeloid-derived suppressor cells associated with severe COVID-19, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adn7527