Every breath we take carries with it an invisible symphony — the molecules of the world around us, drifting into our noses and whispering to our brains. A warm loaf of bread makes us feel comfort and hunger. The scent of a flower evokes calm. A whiff of rot turns our stomachs. Smell is emotion in its purest form — primal, intimate, and immediate.
But how does the brain decide whether a smell is pleasant or repulsive? Is there a single “good versus bad” switch that governs our sense of smell, or is the truth more complex?
A team of researchers in Japan, led by Hokto Kazama at the RIKEN Center for Brain Science (CBS), set out to unravel this mystery. What they discovered fundamentally changes our understanding of how animals — including humans — perceive the world through scent. Their findings, published in the journal Cell, reveal that the brain’s response to odor isn’t simply a scale from pleasant to unpleasant. Instead, these sensations are crafted by entirely separate circuits — two independent systems working side by side, shaping how we experience the world’s fragrances and foulness alike.
The Ancient Sense That Defines Us
The sense of smell, or olfaction, is one of the oldest sensory systems in evolution. Long before eyes saw or ears heard, the first vertebrates were already sensing chemicals in their watery worlds. These primitive chemical detectors helped ancient creatures find food, avoid toxins, and recognize one another — survival coded in scent.
In mammals, including humans, the process is elegant and intricate. Airborne molecules enter the nose and bind to specialized receptors on olfactory neurons. Each receptor detects a range of molecular shapes and sends its signals to the brain, where they are decoded into the experience we recognize as smell.
Yet, this process is far from simple. A single odor is not one molecule but a complex blend. Coffee, for instance, contains more than 800 different aromatic compounds. If each molecule had its own receptor, evolution would never have kept up. Instead, our brains developed a system where thousands of neurons overlap in their responses, creating a unique pattern — like a fingerprint — for every smell.
It’s a brilliant strategy, but also an enormously complicated one. Decoding this overlapping web of activity to understand how the brain determines whether a smell is good or bad is one of neuroscience’s most challenging puzzles.
Why Fruit Flies Hold the Key
When human brains are too complex to study directly, scientists often turn to simpler animals that share similar biological systems. The fruit fly (Drosophila melanogaster) might be tiny, but its brain processes smells in remarkably similar ways to ours. What’s more, scientists can identify every single neuron in a fruit fly’s brain and trace its connections — an impossible task in mammals.
Kazama and his team chose the fruit fly not for its simplicity, but for its accessibility. Despite having only about 100,000 neurons (compared to our 86 billion), the fly’s olfactory system captures the same fundamental logic of how brains process smells. By studying flies, researchers can observe entire circuits in action — a feat that would be unimaginable in a human subject.
Mapping the Brain’s Scent Circuits
The team faced a monumental challenge: recording the activity of every neuron involved in smell perception. To achieve this, they combined two cutting-edge technologies.
Using two-photon microscopy, they could visualize the activity of neurons deep inside the fly’s brain while the insect experienced various odors. They paired this with optogenetics, a revolutionary technique that allows scientists to control neurons with light — turning them on or off with pinpoint precision.
They also created a computational network model — a kind of “digital twin” of the fly brain — built upon the connectome, a complete map of how every neuron connects to every other neuron. This model allowed them to simulate how the brain processes odors and predict what would happen if specific circuits were altered.
Through this intricate approach, the researchers uncovered something extraordinary.
Where Smells Become Feelings
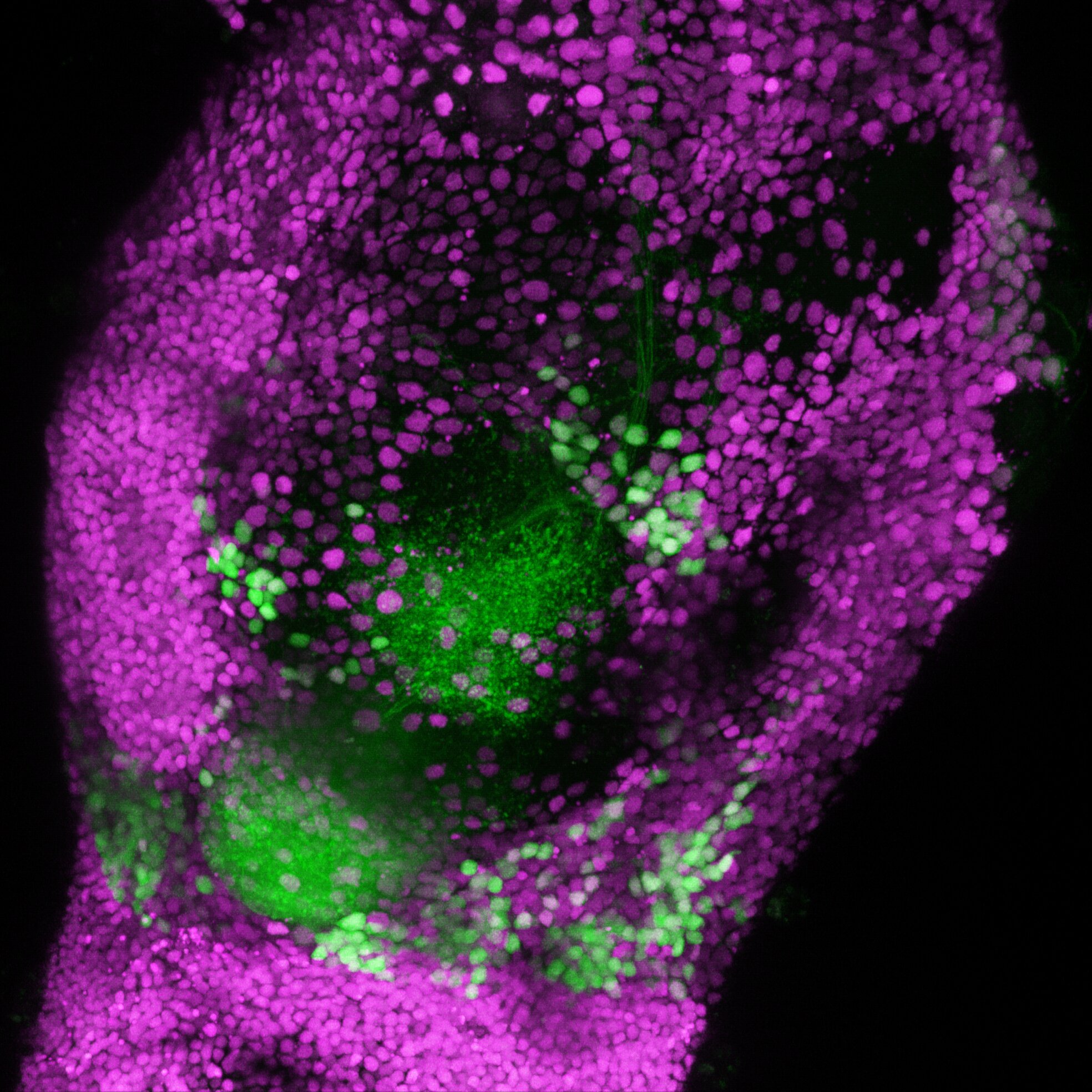
Inside the fly’s brain lies a region called the lateral horn, known for processing innate behaviors — those hardwired instincts that determine whether a fly is drawn to something or repelled by it. It is here that the brain decides if an odor is pleasant or unpleasant.
Kazama’s team discovered that pleasant and unpleasant odors are represented by entirely separate circuits in the lateral horn. In other words, the brain doesn’t judge smell along a single “pleasantness scale.” Instead, it processes “good” and “bad” smells using two distinct sets of neurons — two separate languages of scent.
Even more fascinating, these circuits are not mirror images of each other. Unpleasant smells are handled through a feedforward system — a straightforward pathway that rapidly excites neurons to signal disgust or danger. Pleasant smells, on the other hand, involve an added layer of complexity: local inhibitory circuits that refine and shape the signal.
This finding overturned a long-held assumption. We tend to think of pleasure and disgust as opposites — like light and dark, or hot and cold. But in the brain, they are built differently. “Good” is not the absence of “bad.” They are two separate symphonies playing distinct tunes.
When Light Becomes the Switch for Smell
Once the team’s computational model predicted how the brain should behave, the scientists put it to the test. Using optogenetics, they could shine light on specific neurons and manipulate the circuits in real time.
When they silenced certain neurons predicted to encode pleasantness, the flies began to avoid odors they had previously found attractive. When they activated those same neurons, even normally neutral smells became appealing. The results were strikingly precise — as if they had found the literal switch that determines a fly’s likes and dislikes.
These experiments confirmed that the brain’s perception of smell is not just about detecting molecules — it’s about computing meaning. Every scent is filtered through emotional circuits that give it value: to approach or to avoid, to savor or to escape.
A Window Into the Human Mind
Though this research focused on fruit flies, its implications reach far beyond the insect world. The olfactory system, one of evolution’s most conserved sensory systems, is remarkably similar across species. The way flies process scent offers clues to how mammals — including humans — do the same.
Our brains, too, contain regions that evaluate the emotional significance of smells. The scent of smoke, for instance, instantly triggers alarm; the smell of vanilla soothes. These emotional reactions are among the oldest and most instinctive responses in the brain, deeply tied to memory and survival.
Understanding these mechanisms may one day help scientists decode how the human brain links smells with feelings, memories, and even mental health. Disorders like depression, anxiety, and Alzheimer’s disease are often accompanied by changes in smell perception. By studying these circuits in simpler organisms, researchers may uncover insights that could lead to better diagnostics and therapies.
The Future: From Flies to Artificial Intelligence
Kazama’s work does not stop at biology. The team’s creation of a connectome-based network model — a digital reconstruction of how neurons interact — is a step toward something even more ambitious: building a digital twin of the brain.
Such models could simulate how a brain responds to new experiences, predict its behavior under different conditions, and inspire new technologies in artificial intelligence.
“By implementing the circuit mechanisms we discovered in the compact fly brain,” Kazama explains, “we may be able to develop more efficient algorithms and high-efficiency brain-inspired AI.”
Nature, after all, has been refining its information-processing systems for billions of years. The way a fly distinguishes a sweet fruit from rotting waste could hold the key to designing machines that make nuanced decisions — machines that learn to “smell” opportunity and “avoid” error, just as living brains do.
More information: Makoto Someya et al, Distinct circuit motifs evaluate opposing innate values of odors, Cell (2025). DOI: 10.1016/j.cell.2025.08.032