In the complex architecture of the human body, neurons are the crown jewels of our nervous system—highly specialized cells that power every thought, movement, and sensation. But unlike most other cells, neurons are not known for their ability to recover after damage. When trauma strikes—through a stroke, concussion, or degenerative disease—neurons tend to break down rather than rebuild. Their axons, the long, cable-like fibers that transmit electrical signals across the brain and spinal cord, often deteriorate permanently.
But a groundbreaking study from the University of Michigan, published in Molecular Metabolism, is challenging that bleak narrative. The research, conducted using a fruit fly model, reveals a surprising new role of cellular metabolism—how neurons process sugar—in determining whether these vital cells survive or surrender to degeneration.
The implications are profound. The findings open a new window into understanding how, and why, some neurons resist breakdown while others succumb. Even more promising, this research could lead to innovative therapies for some of the most devastating neurodegenerative conditions, from Alzheimer’s to Parkinson’s to traumatic brain injury.
Not All Damage Is Final—And Sugar May Hold the Key
For decades, scientists have viewed the breakdown of neurons after injury as an inevitable decline, a one-way street. But that view is now being questioned.
“Metabolism is often changed in brain injury and diseases like Alzheimer’s, but we do not know whether this is a cause or consequence of the disease,” explained Dr. Monica Dus, senior author of the study and associate professor of molecular, cellular, and developmental biology at the University of Michigan.
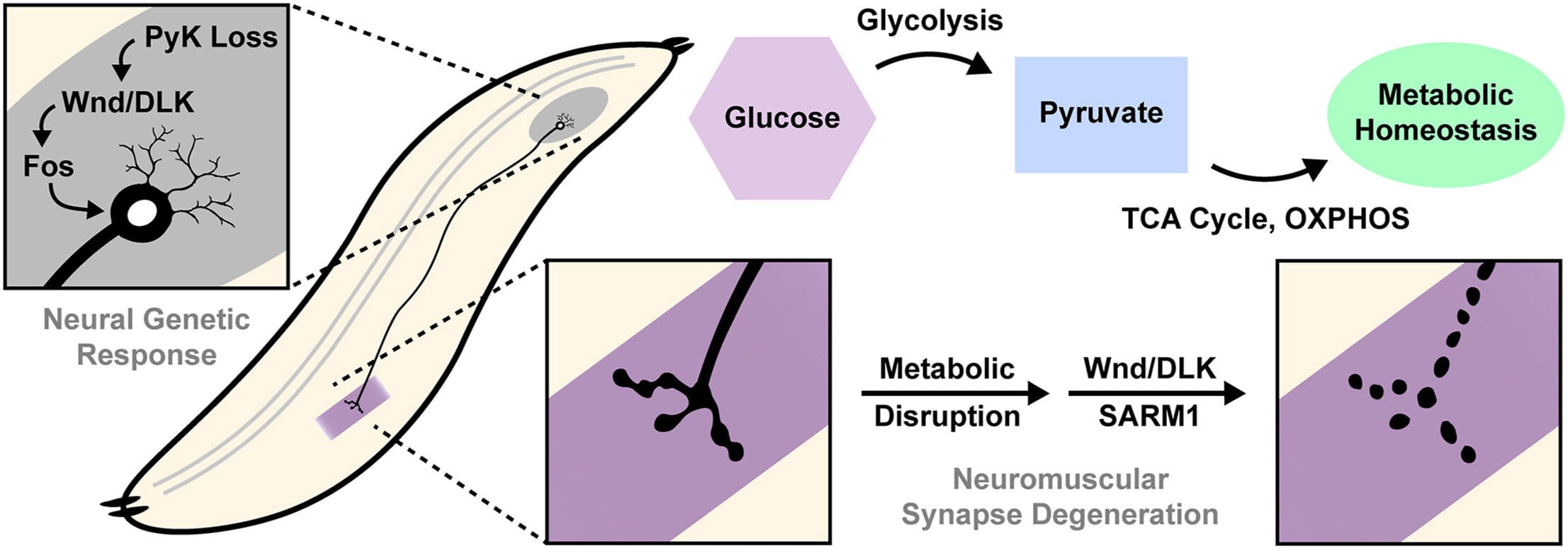
Her team discovered that the way neurons process glucose—the brain’s primary energy source—can determine how resilient they are in the face of injury. Altering the sugar metabolism of neurons had radically different effects depending on the cell’s condition: in healthy neurons, interfering with sugar metabolism hastened axon breakdown; in already-damaged neurons, the same intervention actually triggered a protective response, preserving the axon’s structure.
This contradictory response isn’t just a scientific curiosity—it could be a vital clue to understanding what separates brains that heal from those that deteriorate.
Two Proteins, One Crucial Crossroads
The study zeroed in on two key proteins: DLK (dual leucine zipper kinase) and SARM1 (Sterile Alpha and TIR Motif-containing 1). These molecules aren’t household names, but they may be crucial to the future of neurodegenerative disease treatment.
DLK acts as a kind of damage sensor. When neurons are injured or metabolically stressed, DLK activates. This activation then triggers a cascade of cellular responses. One of those responses involves SARM1, which is well-known for its role in initiating axon degeneration. When overactivated, SARM1 leads to the death of the axon—like pulling the plug on the brain’s internal wiring.
But here’s where things get fascinating: when glucose metabolism was dialed down in already-injured neurons, the study found that DLK was activated in a way that suppressed SARM1, effectively protecting the axon from further degradation. Instead of dying, the axons endured.
“This is a new perspective on injury and disease,” said Dus. “It’s not just about blocking damage—it’s about understanding what the system is already doing to try to protect itself and then reinforcing that effort.”
When Protection Becomes Destruction
However, the story doesn’t end with a happy twist. Like many mechanisms in biology, this one is a double-edged sword.
While DLK activation can initially protect neurons, prolonged activation leads to the opposite effect: progressive degeneration. It’s as if DLK has two faces—one that fights for survival, and one that signals surrender. And knowing when, and how, DLK flips that internal switch could be the holy grail for treating conditions like ALS, multiple sclerosis, and Alzheimer’s.
TJ Waller, postdoctoral research fellow and lead author of the study, likens it to controlling a fire. “If we want to delay the progression of a disease,” he said, “we want to inhibit its negative aspect. But we also need to make sure we’re not inhibiting the more positive aspect that might actually be helping to slow the disease down naturally.”
This balancing act—activating DLK just enough to initiate repair, but not so much that it causes collapse—is the central puzzle that researchers now face. Cracking that code could give doctors a powerful new tool to slow or even halt brain degeneration.
Why Fruit Flies Might Save Your Brain
The research team used fruit flies as their model organism—a choice that may sound simple, but is actually one of neuroscience’s greatest secrets. Despite their tiny size and short lifespan, fruit flies share many of the same genetic and cellular pathways as humans, especially when it comes to the nervous system.
In fact, discoveries in fruit fly models have historically led to breakthroughs in understanding human diseases, including cancer, heart conditions, and neurological disorders. The advantage lies in their speed: experiments that would take years in mice can be done in weeks in flies. And the precision with which scientists can manipulate genes in flies is unparalleled.
By using this model, Dus and her team were able to trace the intricate dance between sugar metabolism, DLK activation, and axon survival in real-time. Their work represents one of the first efforts to directly link glucose metabolism to the molecular machinery of neuronal resilience.
From Bench to Bedside: What This Means for Patients
The potential real-world applications of this research are enormous. In diseases like Alzheimer’s and Parkinson’s, the slow, progressive loss of neurons is the root cause of cognitive and motor decline. In traumatic injuries, like spinal cord damage or stroke, neurons often die off in the days and weeks following the event—not just at the moment of trauma.
If scientists can find a way to manipulate DLK’s dual nature—preserving its protective effects while neutralizing its harmful ones—it could open a therapeutic window for saving neurons even after injury has begun.
“These findings suggest that there is a natural mechanism in place that is already trying to defend the nervous system,” said Waller. “If we can understand and amplify that response, we might finally be able to move beyond just managing symptoms and start protecting or even healing the nervous system itself.”
For now, these insights are still early-stage. Much more research is needed to translate them into human therapies. But this study provides a clear, scientifically plausible target—and a reason for hope.
A New Way of Thinking About Brain Health
This research does more than identify a new potential treatment pathway—it shifts the way scientists think about the nervous system’s capacity for self-repair. Rather than viewing the brain as helpless in the face of injury or disease, Dus and her colleagues see a more dynamic, responsive system—one that fights to survive, one that may just need a little help.
Their findings suggest that brain degeneration isn’t just about what goes wrong; it’s also about what tries to go right and fails. If we can learn how to support those internal rescue efforts—boosting the body’s own protective programs while minimizing self-destructive ones—we might uncover entirely new strategies for defending the most complex organ we possess.
In a world facing a growing burden of neurodegenerative disease, this kind of thinking is not just revolutionary. It’s essential.
Funding, Support, and the Road Ahead
The research was supported by the National Institutes of Health, the U.S. National Science Foundation, the Rita Allen Foundation, and the Klingenstein Fellowship in the Neurosciences—a testament to how collaborative and cross-disciplinary efforts continue to drive medical science forward.
As scientists now race to untangle DLK’s molecular behavior and its interaction with sugar metabolism, new questions arise: Can drugs be designed to harness this protective effect without triggering degeneration? Are there specific time windows after injury when these interventions are most effective? Could metabolic therapies—perhaps even diet-based interventions—help reinforce neuron survival in patients at risk?
The University of Michigan team remains optimistic, if cautious. “There’s still so much we don’t know,” Dus said. “But what’s clear is that neurons are not passive victims. They’re trying to survive. We just need to learn how to help them succeed.”
Conclusion: A Spark in the Darkness
Neurodegeneration has long been a domain of despair—a slow unraveling that medicine struggles to delay, let alone stop. But the work of Dus, Waller, and their team offers a different vision. It’s a story of molecular courage, of neurons that try to hold the line even in crisis, and of scientists who dare to listen to what those cells are saying.
In the tiny synapses of a fruit fly brain, researchers have glimpsed a truth that could one day help millions. That’s the power of science—not just to understand, but to transform, to protect, and perhaps, in time, to heal.
More information: Thomas J. Waller et al, Pyruvate kinase deficiency links metabolic perturbations to neurodegeneration and axonal protection, Molecular Metabolism (2025). DOI: 10.1016/j.molmet.2025.102187