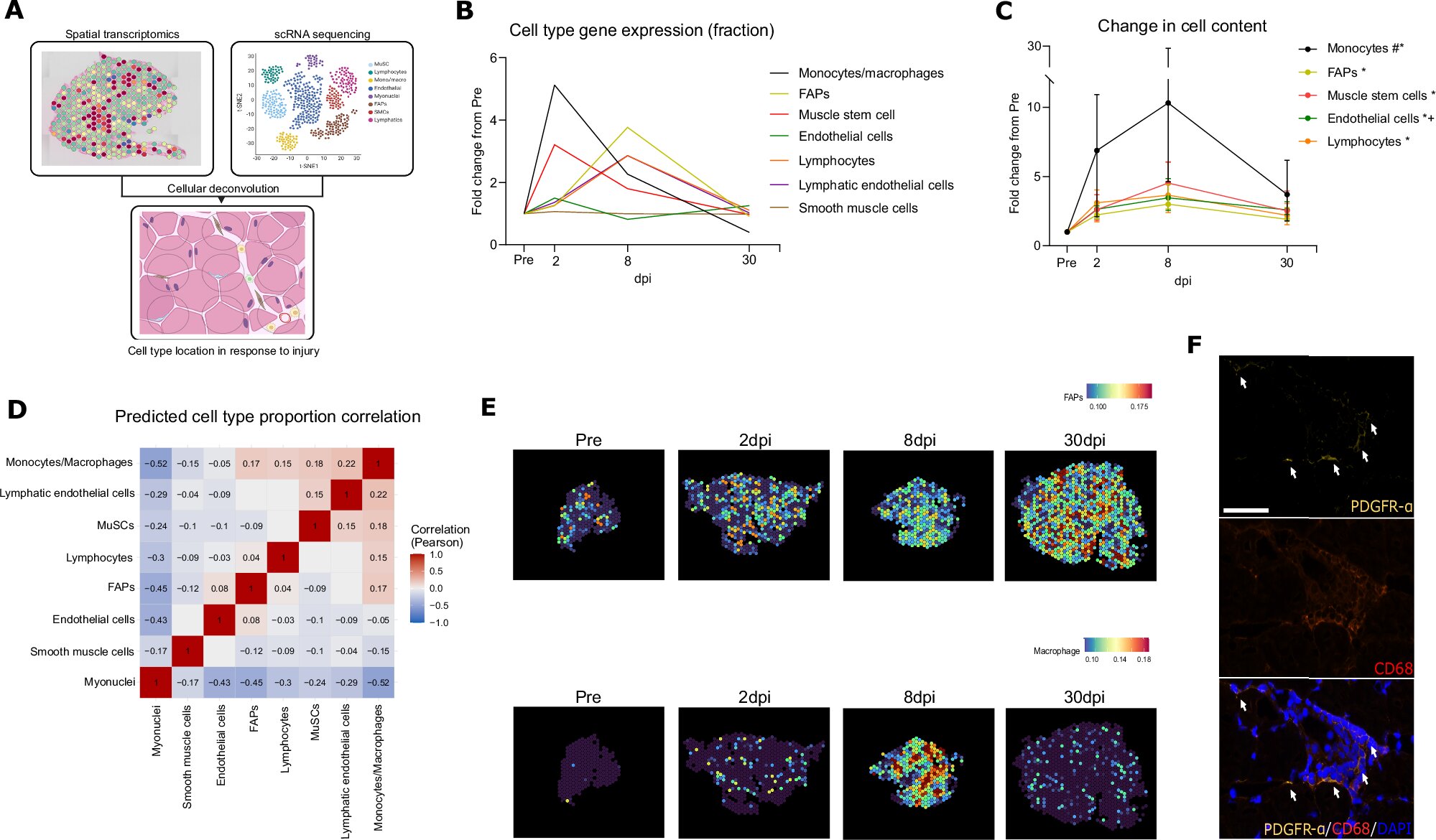
When a storm tears through a house, the rebuilding doesn’t begin until someone clears out the broken beams, shattered glass, and debris. Only then can the work of reconstruction truly start. Inside the human body, a similar process unfolds every time our muscles are injured—whether from intense exercise, illness, or aging. But until now, scientists didn’t fully understand who leads that internal clean-up crew.
Now, a groundbreaking discovery from researchers at Aarhus University and Steno Diabetes Center Aarhus has revealed a surprising answer: a little-known group of cells called fibro-adipogenic progenitors (FAPs) serve as coordinators of muscle repair, orchestrating a delicate conversation between immune cells and muscle tissue to guide healing.
Published in Nature Communications, this new study not only unlocks a hidden chapter of muscle biology but also opens promising doors for treating age-related muscle loss, chronic diseases, and injury recovery.
The Hidden Conductors of Muscle Healing
Every muscle injury—no matter how small—triggers a symphony of cellular responses. But instead of random chaos, healing follows a highly choreographed performance. The new research shows that FAPs act like project managers, relaying critical instructions to the immune system’s “construction crew” of macrophages—cells that gobble up damaged tissue and help kickstart regeneration.
“FAPs are a kind of coordinator that lives in the house and knows how it should be rebuilt,” explains Associate Professor Jean Farup, who led the study. “They communicate with the various craftsmen necessary for the reconstruction.”
That communication, the team found, is not just metaphorical. It’s biochemical, involving precise molecular signals that influence the entire repair process.
An Unexpected Source of a Powerful Signal
Perhaps the most surprising discovery came when the researchers traced the source of complement C3, a key signaling molecule traditionally believed to be produced in the liver and circulated through the blood.
Not so fast.
The team discovered that FAPs themselves produce complement C3—right inside the muscle tissue. This local production isn’t just convenient. It’s vital.
Complement C3 helps regulate inflammation, a necessary phase in muscle repair. While excessive or chronic inflammation can be damaging, a well-controlled inflammatory response clears out damaged cells and creates the right environment for muscle regrowth. The FAPs’ local release of complement C3 fine-tunes this balance, ensuring that macrophages receive just the right instructions at just the right time.
This direct, localized signaling rewrites much of what scientists previously assumed about muscle regeneration—and it helps explain why healing sometimes goes wrong when inflammation isn’t well controlled.
Hope for the Aging Body
For many people, this discovery couldn’t come at a more important time. As we age, our muscles naturally lose mass and strength—a condition known as sarcopenia. It’s not just a cosmetic issue; it affects mobility, independence, and quality of life.
Moreover, chronic illnesses such as type 2 diabetes, cardiovascular disease, and cancer are often accompanied by muscle wasting. In these cases, the inflammation that’s normally helpful becomes prolonged and damaging, preventing proper muscle regeneration.
“We know that inflammation plays a significant role both in relation to muscle regeneration and in connection with muscle mass loss in patients and elderly people,” says Farup. “Our new insight therefore provides new opportunities to regulate inflammation with, for example, medicine that affects FAPs.”
In other words, this discovery isn’t just about understanding how muscles heal. It’s about figuring out how to support that process when it fails—and intervene with precision.
Rewriting the Blueprint for Muscle Therapies
Until now, therapies aimed at muscle loss have largely focused on stimulating muscle growth directly—through exercise, nutrition, or anabolic drugs. But this study suggests another approach: tuning the conversation between FAPs and immune cells.
If scientists can harness or mimic the local signaling pathways used by FAPs, it might be possible to enhance the body’s own healing processes—especially in people whose inflammation is stuck in the “on” position.
The researchers are already planning to test whether this same communication mechanism is altered in conditions characterized by chronic inflammation. Do FAPs fail to produce the right signals? Do immune cells stop listening? Are there ways to reset this internal messaging system?
These questions are now at the forefront of muscle biology.
The Cell You’ve Never Heard of May Hold the Key
FAPs are hardly household names. Until recently, their role in muscle tissue was considered secondary. They were sometimes dismissed as passive support cells or linked to fat accumulation in diseased muscle.
But this new study elevates their status dramatically, placing them at the heart of a finely tuned repair system. They don’t just watch the clean-up crew work. They call them in, tell them where to go, and provide the chemical cues to get the job done.
Their ability to produce complement C3 locally is a game-changer in how we understand immune regulation in muscle tissue. It adds a new layer to our understanding of the immune-muscle interface, a realm of growing importance in both regenerative medicine and immunology.
A New Chapter in the Science of Healing
This research marks a turning point—not just in our understanding of muscle regeneration, but in how we view the entire ecosystem of cells involved in healing. Where once we saw muscle fibers and immune cells as the main players, we now recognize FAPs as a kind of cellular foreman, orchestrating the response with molecular precision.
And this matters. Not just for athletes nursing injuries or the elderly striving to stay mobile—but for millions of people worldwide whose bodies are caught in a tug-of-war between healing and inflammation.
This discovery might be the first step toward new therapies that don’t just stimulate growth, but reignite the body’s own intelligence to heal—guided by the cells that have been quietly coordinating all along.
As Farup and his team continue to explore how FAPs operate in chronic disease, one thing is clear: the path to better muscle health lies not just in building stronger muscles, but in understanding the messengers that tell them when and how to grow.
Reference: Jonas Brorson et al, Complementing muscle regeneration—fibro-adipogenic progenitor and macrophage-mediated repair of elderly human skeletal muscle, Nature Communications (2025). DOI: 10.1038/s41467-025-60627-2