In a brightly lit laboratory at Toronto’s Hospital for Sick Children, scientists are rewriting the rules of cancer diagnostics. With targeted RNA sequencing, they’ve discovered a way to uncover hidden genetic signals in tumors—signals that traditional DNA methods often miss. For patients and families navigating the fear and uncertainty of cancer, this breakthrough offers something priceless: clearer answers, faster treatments, and renewed hope.
Published in Nature Medicine, the study demonstrates that RNA sequencing can detect clinically actionable genetic alterations in an astonishing 87% of tumors. That means doctors now have a more powerful tool to guide personalized therapies, even in cases where DNA tests fail to reveal what’s driving the cancer.
Why RNA Matters in the Fight Against Cancer
For decades, DNA-based diagnostics have been the backbone of molecular cancer testing. These methods look for mutations—single-letter changes, insertions, deletions—that can explain why healthy cells spiral into uncontrolled growth. But DNA testing has limitations. It struggles to detect complex genetic rearrangements known as fusions and can’t always determine the real-world impact of mutations on how genes are expressed.
RNA sequencing changes that equation. Instead of looking only at the static blueprint of DNA, it examines the active instructions—RNA—that cells use to build proteins. This approach allows scientists to see, with exquisite sensitivity, when two genes fuse abnormally or when the normal processing of RNA (splicing) goes awry. These insights can make the difference between a vague diagnosis and a precise, actionable treatment plan.
The Scope and Scale of the Study
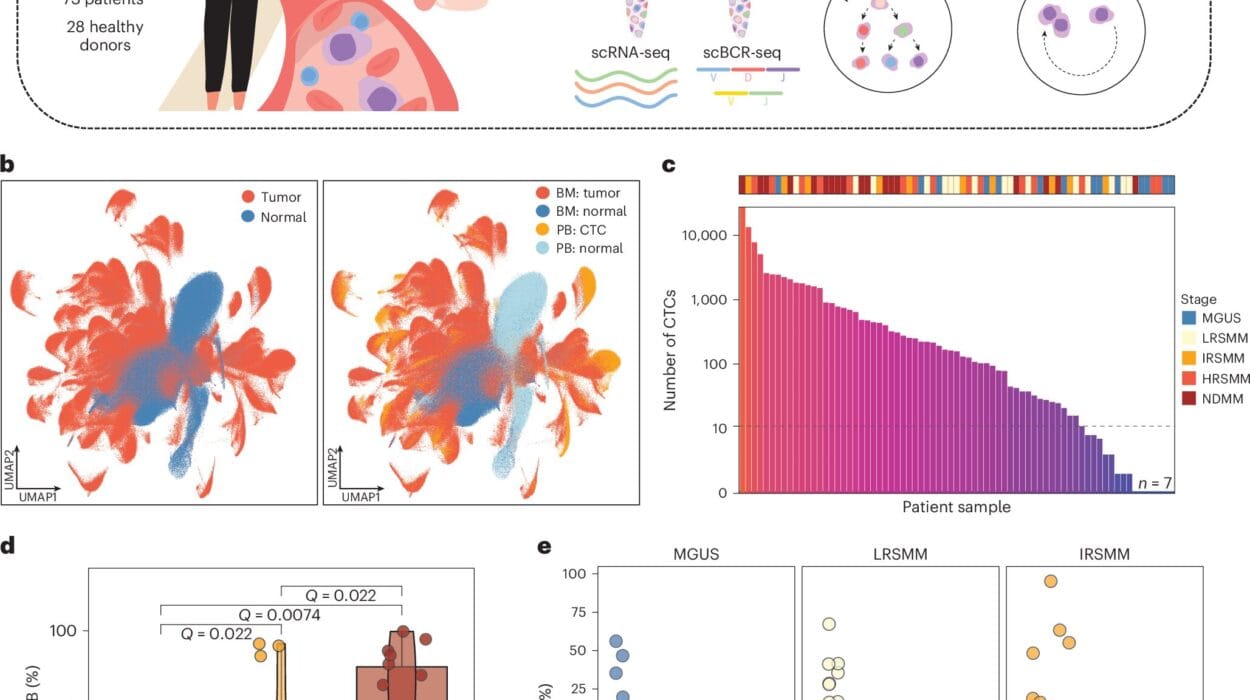
To understand how well RNA sequencing works in a clinical setting, researchers analyzed 2,310 tumors from children and adults with cancers of the brain, blood, and other solid organs. Despite the challenges of working with fragile RNA, the assay achieved a remarkable success rate: 99.6% of samples that passed quality checks were successfully sequenced.
The findings were transformative. Oncogenic variants—genetic changes that promote cancer—were detected in 74% of tumors. Another 13% were classified as “pertinent negatives,” meaning no actionable changes were found but the negative result was clinically meaningful. Together, this represents a total clinical utility of 87%, far surpassing traditional DNA-only methods.
RNA sequencing identified different types of alterations: single nucleotide changes and small insertions or deletions in 40% of cases, gene fusions in 32%, structural variants in 2.5%, and multiple variant classes in 5%. Interestingly, fusions were significantly more common in pediatric tumors, while single nucleotide changes dominated in adults—information that could help tailor future testing strategies.
A New Lens on Tumor Identity
Sometimes, cancer is misclassified based on how it looks under a microscope. RNA sequencing provided a sharper lens, allowing doctors to revise diagnoses in 48 patients. For 37 of these, RNA findings revealed an entirely new diagnosis; for 11 others, the presence or absence of certain alterations led to reclassification.
Central nervous system tumors accounted for most of these revised diagnoses. In 13 cases, what was initially believed to be ependymoma turned out to be glioma, astroblastoma, or paraganglioma. Seven low-grade gliomas were reclassified as a more aggressive type, diffuse midline glioma, H3 K27-altered, thanks to the detection of H3K27M mutations—changes invisible to DNA sequencing alone.
One especially striking case involved a tumor initially diagnosed as metastatic Wilms tumor. RNA sequencing revealed a BCOR internal tandem duplication, identifying it instead as a clear cell sarcoma of the kidney. In another case, RNA testing uncovered a classic COL1A1::PDGFB fusion, transforming a negative test result into a definitive diagnosis of dermatofibrosarcoma protuberans.
These aren’t just scientific footnotes—they’re stories of real patients whose treatment paths shifted dramatically thanks to this technology.
From Data to Decisions
Precision diagnostics matter only if they lead to better treatments. In this study, 94 of 104 patients evaluated for targeted therapy went on to receive treatment based on RNA findings. Many received therapies that directly attack the biological pathways fueling their cancer, such as MAPK pathway inhibitors, tyrosine kinase inhibitors, and immune checkpoint therapies. For families facing the most daunting of diagnoses, each new therapy represents a new lifeline.
One of the most compelling discoveries was that in fusion-driven tumors—where two genes abnormally join together—these fusions alone explained cancer development in 86% of cases. Unlike other tumors that accumulate multiple mutations, these cancers had a single molecular “spark,” making them prime candidates for therapies that specifically target fusion proteins.
A Tool That Works Across Tumors and Time
Another advantage of RNA sequencing is its versatility. It succeeded across a wide range of tumor types and worked even with degraded samples preserved in formalin-fixed paraffin-embedded (FFPE) tissue, a common archival method in pathology labs. This means hospitals could potentially apply RNA testing to existing biopsy material, without needing fresh tissue or repeat procedures.
Comparisons with matched DNA sequencing further underscored RNA’s value. Of 103 samples with both tests, RNA sequencing detected 93.3% of the same oncogenic variants, with strong agreement in allele frequencies. But crucially, RNA uncovered additional, clinically relevant changes in cases where DNA alone returned no findings or ambiguous results.
The Future of Cancer Diagnostics
The study’s authors conclude that targeted RNA sequencing is more than a complement to DNA testing—it can stand alone as a primary molecular diagnostic tool. With high sensitivity, broad applicability, and relatively low cost, RNA testing could become a cornerstone of precision oncology.
For clinicians, it provides a clearer map of cancer’s genetic landscape. For researchers, it offers a trove of data to explore how tumors evolve and respond to treatment. And for patients and families, it means answers where before there were none.
In the words of the researchers, “Targeted RNA-seq can serve as a stand-alone molecular diagnostic with high yield, minimized cost, and broad clinical applicability.” It’s a quiet revolution in cancer medicine, one built not on more powerful microscopes or larger machines, but on listening more closely to the molecular whispers inside every tumor.
As this technology spreads from specialized labs to hospitals worldwide, it could redefine how we diagnose and treat cancer—transforming moments of uncertainty into opportunities for healing, and turning hidden genetic signals into guiding lights for survival.
Reference: Robert Siddaway et al, Clinical utility of targeted RNA sequencing in cancer molecular diagnostics, Nature Medicine (2025). DOI: 10.1038/s41591-025-03848-8