For generations, the human heart has defied one of the most basic principles of biology: the ability to heal itself. Unlike the skin that knits back together after a cut or the liver that regrows after injury, the heart bears its wounds in silence. Once damaged—especially by a heart attack—it is never quite the same. Heart muscle cells, or cardiomyocytes, die and are replaced not by new muscle, but by scar tissue. This loss is permanent. And it leads to a slow, suffocating condition known as chronic heart failure.
But what if that weren’t the end of the story?
In a remarkable leap forward, scientists at the Lewis Katz School of Medicine at Temple University have found a way to possibly reawaken the heart’s regenerative potential. By delivering a developmental gene called PSAT1 through modified messenger RNA—a technology that rose to fame during the COVID-19 pandemic—researchers were able to stimulate heart muscle regeneration in mice who had suffered heart attacks. The results, published in the journal Theranostics, have the potential to redefine how we treat one of the deadliest diseases on the planet.
A Silent Gene With a Powerful Message
In the earliest stages of life, our hearts are astonishingly plastic. The fetal heart has the ability to grow and repair itself as it forms, driven by a symphony of active genes that disappear soon after birth. Among these is PSAT1, a gene deeply involved in the production of serine—a simple but critical amino acid that powers numerous biological pathways.
“In the adult heart, PSAT1 is essentially switched off,” said Dr. Raj Kishore, the study’s senior author and Chair of Cardiovascular Sciences at Temple. “But during development, it plays a major role in supporting rapid cell growth, protecting cells from stress, and helping build blood vessels. We asked: what if we could turn it back on?”
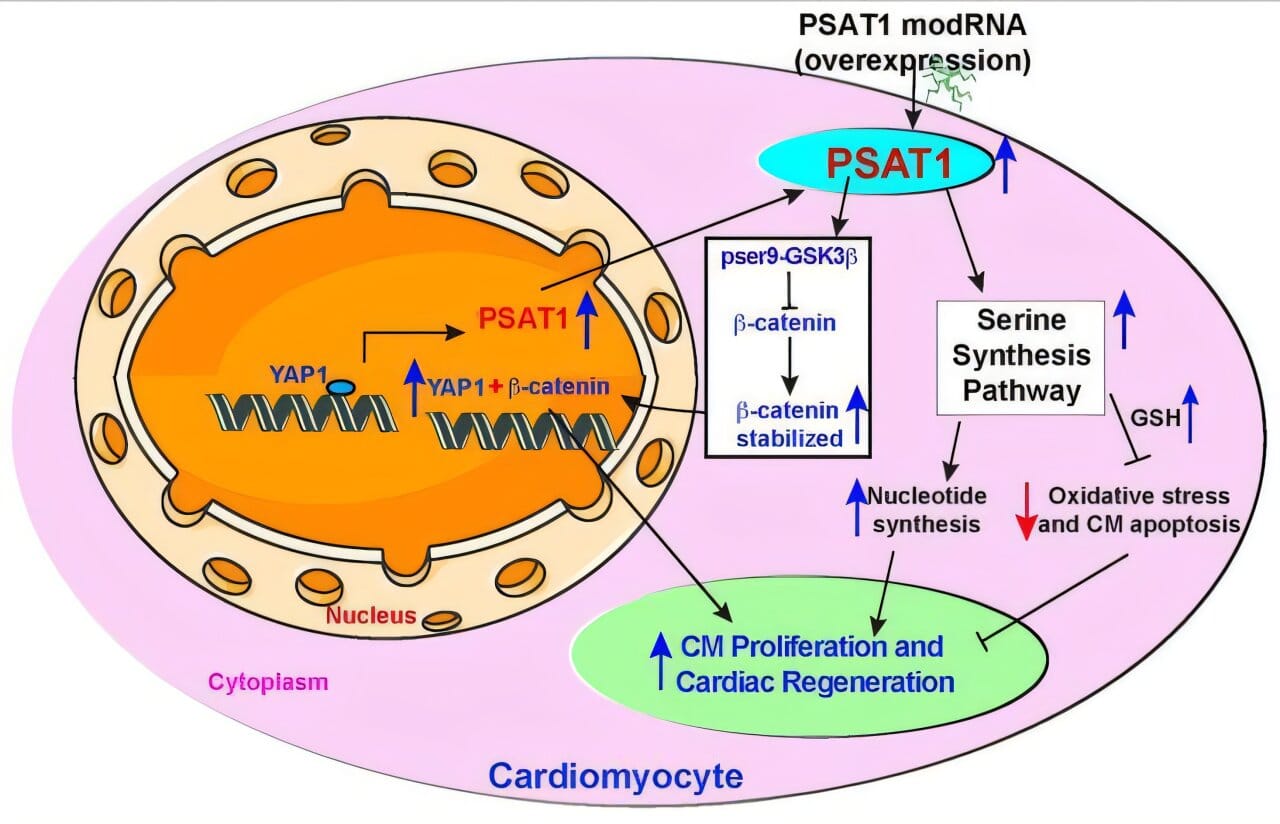
Using synthetic modified mRNA (modRNA), Dr. Kishore’s team did just that. They engineered modRNA to deliver PSAT1 directly into the damaged hearts of adult mice—right at the moment a heart attack occurred. This approach temporarily reactivated PSAT1, waking up genetic programs long considered dormant and inaccessible.
From Scar to Survival: The Impact of PSAT1
The outcome was more than promising—it was revolutionary. In the treated mice, the heart didn’t just survive the injury. It began to heal. Researchers observed a cascade of effects rarely seen in adult hearts:
- Cardiomyocytes started dividing again, showing signs of proliferation.
- Scar tissue, which typically stiffens and chokes the heart, was reduced.
- New blood vessels sprouted in the injured area, restoring oxygen flow and function.
- Most strikingly, the animals experienced improved heart performance and survival rates.
This wasn’t just biological patchwork. It was regeneration.
Inside the Machinery: How PSAT1 Works
To understand how a single gene could produce such sweeping effects, the team dug deeper. They found that PSAT1 activates something called the serine synthesis pathway (SSP). While this pathway is often overlooked in adult tissues, it is vital for rapidly growing cells. SSP helps supply cells with nucleotides for DNA replication and supports antioxidant defenses that fend off damaging stress.
After a heart attack, cells in the heart are bombarded with oxidative stress and DNA damage—two major culprits in cardiomyocyte death. By turning on SSP, PSAT1 helped cells survive longer, divide more frequently, and function more robustly.
Even more intriguing was the discovery that PSAT1 works in concert with other molecular players. The gene is turned on by YAP1, a protein known for promoting regeneration in multiple tissues. Once active, PSAT1 also encourages β-catenin to enter the nucleus—a critical step for pushing cardiomyocytes back into the cell cycle and triggering repair.
“This isn’t just one switch flipping on,” Dr. Kishore explained. “It’s a master circuit board being powered up—activating multiple programs that work together to regenerate the heart.”
The Messenger That Changes Everything: Why modRNA Matters
Central to this breakthrough is the use of modRNA, a modified version of messenger RNA that instructs cells to produce specific proteins. modRNA has two major advantages over traditional gene therapy.
First, it is non-integrative. Unlike viral gene therapy, which can insert DNA into the genome (sometimes in unwanted places), modRNA does not alter the patient’s DNA. This reduces the risk of causing mutations or cancerous growths.
Second, it is temporary and tunable. modRNA is degraded naturally over time, allowing researchers to control how long and how much of a protein is produced. This fine-tuning is critical for powerful genes like PSAT1, where too much activity could cause unintended consequences.
The success of mRNA vaccines during the COVID-19 pandemic has accelerated research into RNA-based therapeutics. With this study, the heart—long considered an organ beyond repair—has entered the conversation.
From Mice to Humans: A Long Road Ahead
Despite the stunning results in animal models, PSAT1 therapy is still in its early stages. The researchers now face the daunting task of translating these findings into safe, effective treatments for people.
“We need to understand how this therapy performs in larger animals whose hearts are more like ours,” said Dr. Kishore. “And we have to refine how and when the gene is delivered. Timing is everything in cardiac regeneration.”
In particular, the team is exploring more precise control over gene expression—ensuring that PSAT1 is activated only in the damaged area and only for as long as needed. They are also working on delivery systems that could reach the heart noninvasively, possibly through intravenous injection or catheter-based approaches used in existing heart procedures.
Another challenge lies in the complexity of human disease. Heart attacks rarely occur in isolation. Most patients suffer from diabetes, high blood pressure, or chronic inflammation—all of which could interfere with the regenerative response.
But despite these hurdles, the potential is undeniable.
A Future Without Irreversible Heart Damage?
For decades, heart failure has been a sentence, not a symptom. It creeps into a patient’s life slowly after the trauma of a heart attack, limiting activity, shortening breath, and eventually, life itself. The best current treatments—like medications, implanted devices, and transplants—can manage the damage, but they cannot undo it.
The PSAT1-modRNA therapy hints at a different vision: a heart that heals.
It suggests that the human body, even in adulthood, might retain ancient capacities for repair—if only we can find the right key. PSAT1 appears to be one of those keys. Not a miracle, but a memory from our developmental past, reawakened by modern science.
“This work is about more than fixing a broken pump,” said Dr. Kishore. “It’s about restoring the very essence of what it means to be alive—to repair ourselves, to regenerate, to survive what would once have been fatal.”
Hope Written in Code
In the molecular language of biology, every gene carries a story. PSAT1 tells the story of early growth, of embryonic development, of life rapidly assembling itself from atoms and energy. Now, with the help of synthetic RNA, that story can be told again—not in a womb, but in a heart fighting to beat after trauma.
As research continues and clinical trials inch closer, the implications ripple far beyond cardiology. Could modRNA therapies be used to heal damaged kidneys, lungs, or even brains? Could the tools of development become the instruments of healing for adults across all ages?
Those questions remain. But one thing is clear: the future of regenerative medicine is no longer science fiction. It is scientific fact, emerging from the lab and beating within the hearts of mice who once had none left to give.
And someday, it may beat within us, too.
More information: Ajit Magadum et al, Phosphoserine aminotransferase 1 promotes serine synthesis pathway and cardiac repair after myocardial infarction, Theranostics (2025). DOI: 10.7150/thno.112077