Since early 2024, the highly pathogenic avian influenza strain H5N1 has swept through U.S. poultry farms, wild bird populations, and—more unusually—dairy cattle. For decades, scientists have worried that this virus, carried silently by migratory birds but deadly in domesticated animals, could someday leap more easily into humans. That worry now feels urgent. More than 70 people in the United States have already been infected, primarily through contact with sick livestock, and at least one person has died.
H5N1 infections are severe, often fatal, and experts fear the virus could evolve to spread from person to person, igniting a pandemic. In response, researchers around the world are racing to understand how this virus attacks the body and to create vaccines that could stop it in its tracks.
At the Texas Biomedical Research Institute (Texas Biomed), two different but interconnected research efforts are offering hope. One team is creating a next-generation vaccine, while another is uncovering how the virus damages human airways—and pointing to new ways to fight back.
A New Kind of Bird Flu Vaccine
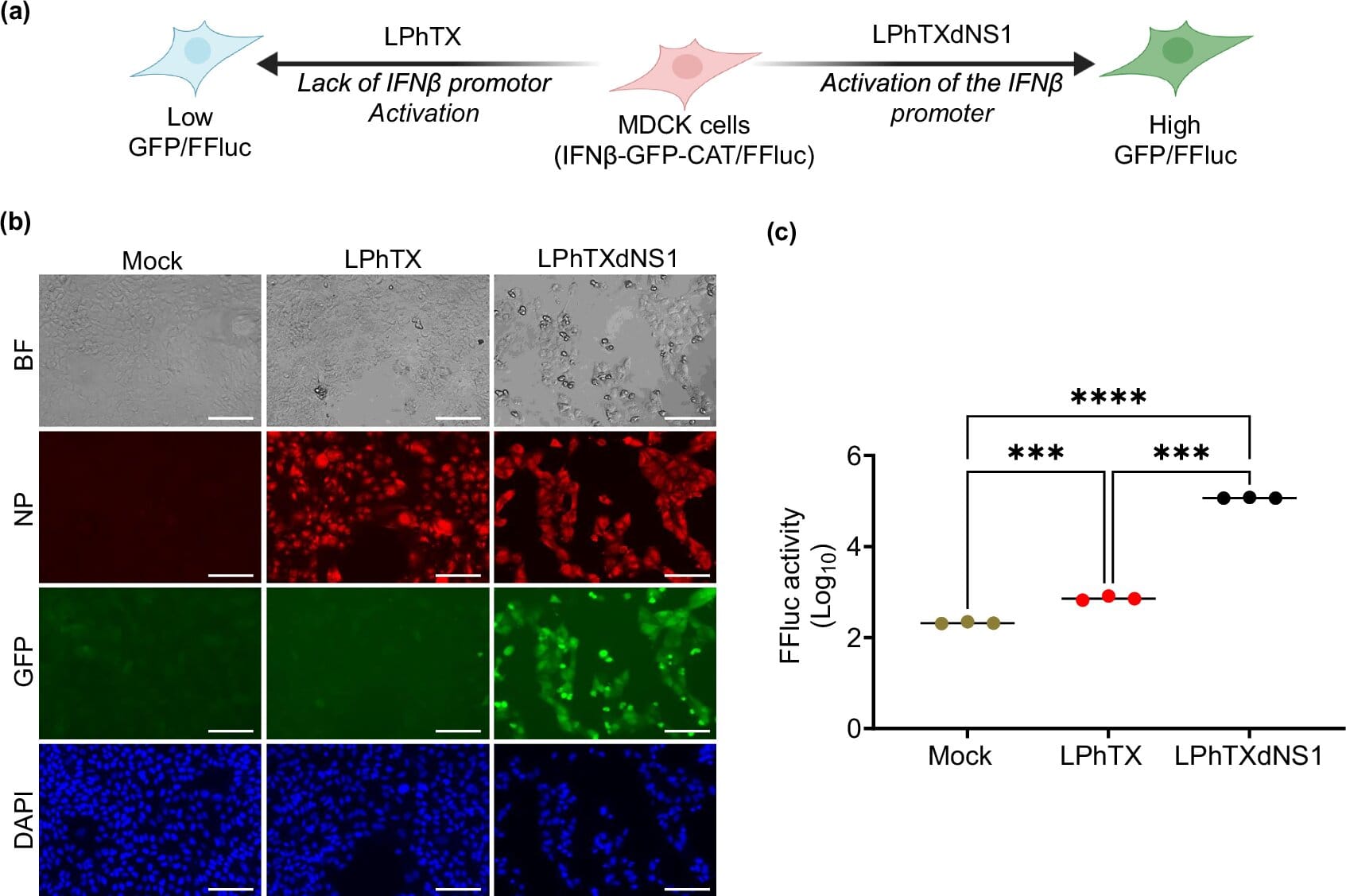
In a recent study published in npj Vaccines, a team led by Professor Luis Martinez-Sobrido, Ph.D., and Staff Scientist Ahmed M. Elsayed, Ph.D., unveiled a live attenuated vaccine designed to protect against the current H5N1 strain. Unlike older vaccines made from inactivated, or “killed,” viruses, this vaccine uses a weakened version of the bird flu virus itself.
The concept is simple but powerful: by exposing the immune system to a live, harmless version of H5N1, the vaccine triggers a stronger and longer-lasting defense. In laboratory tests, a single dose of the vaccine was enough to protect mice from otherwise lethal exposure to H5N1.
“We know that live attenuated vaccines tend to give more robust protection,” Dr. Martinez-Sobrido explained. “They mimic a natural infection much more closely, so the immune system responds more effectively.”
The goal now is to build on this success. The researchers are working to adapt their vaccine platform to target multiple bird flu strains circulating worldwide. Dr. Elsayed is optimistic: “The ultimate aim is to develop a vaccine that could protect against multiple strains of bird flu, or even offer universal protection.”
Why This Matters
The United States already has emergency stockpiles of bird flu vaccines, but those are based on older virus strains and use traditional inactivated virus technology. They do work, but they may not provide the same level of durability or breadth of protection as a live attenuated vaccine.
Given that H5N1 has already infected mammals ranging from sea lions to cats—and now cattle—the possibility of further viral evolution is real. If the virus ever acquires the ability to spread efficiently between humans, having the best possible vaccines ready could save countless lives.
How Bird Flu Damages the Airways
While one group at Texas Biomed focuses on prevention, another team is uncovering how H5N1 wreaks havoc inside the body. In a study published in Emerging Microbes & Infections, Staff Scientist Hussin Rothan, Ph.D., and Dr. Martinez-Sobrido’s group turned to human airway organoids to model infection.
Organoids are miniature, three-dimensional models of human tissues grown in the lab. These airway organoids include the same four key cell types that line the trachea, complete with cilia that wave back and forth and mucus-producing cells. In other words, they recreate a living, breathing slice of the human airway.
When exposed to H5N1 taken from infected dairy cattle, these organoids showed dramatic changes. The virus triggered an intense inflammatory response, leading to a flood of signaling proteins called cytokines. Most concerning, the infection caused the development of fibrotic, or scar-like, tissue in the airway lining—changes that could permanently damage lung function if they occurred in a patient.
The team compared this to infections with seasonal flu viruses like H1N1. The difference was stark: H5N1 caused far more severe damage and inflammation than typical flu.
A New Target for Treatment
In trying to understand this excessive inflammation, the scientists identified a key pathway driving the damage: ROCK, a molecular signaling system involved in tissue remodeling and scarring. To their surprise, they found that inhibiting one enzyme in the pathway, called ROCK1, was far more effective at reducing scarring than blocking its counterpart, ROCK2.
“This was unexpected,” Dr. Rothan explained. “Most of the focus has been on ROCK2. But our results show ROCK1 might be the more critical player when it comes to bird flu and lung damage.”
The finding suggests that therapies targeting ROCK1 could help reduce harmful scarring and inflammation not only in H5N1 infections but also in other respiratory diseases like COVID-19. It opens a new line of investigation into how we might protect the lungs during severe viral infections.
Two Fronts in the Same Fight
Together, these breakthroughs highlight the multi-pronged battle against bird flu. On one hand, vaccines are essential to prevent infections and stop outbreaks before they spiral into pandemics. On the other, understanding how the virus damages human tissues can help doctors treat those who do fall ill.
Both efforts are urgent, because H5N1 is no longer a distant threat. It is here, spreading in U.S. livestock, and occasionally infecting humans. While the virus has not yet learned to spread efficiently between people, evolution is a constant, and the stakes are enormous.
Looking Ahead
The Texas Biomed teams are not alone in this fight, but their work represents important progress. The live attenuated vaccine could become a foundation for stronger, broader, and potentially universal bird flu protection. Meanwhile, the organoid studies point to new treatment strategies that could protect the lungs and save lives during outbreaks.
In the words of Dr. Martinez-Sobrido, “Every step we take brings us closer to being ready—not just for this outbreak, but for whatever influenza may throw at us next.”
For now, the world watches, waits, and prepares. Science cannot eliminate uncertainty, but as this research shows, it can give us tools—powerful ones—to protect ourselves against one of nature’s most dangerous viruses.
More information: Ahmed Mostafa et al, A live attenuated NS1-deficient vaccine candidate for cattle-origin influenza A (H5N1) clade 2.3.4.4.b viruses, npj Vaccines (2025). DOI: 10.1038/s41541-025-01207-9
Hussin Rothan et al, Emerging highly pathogenic H5N1 influenza triggers fibrotic remodeling in human airway organoids, Emerging Microbes & Infections (2025). DOI: 10.1080/22221751.2025.2532684