For millions around the world, inflammatory bowel disease (IBD) is a life lived in quiet agony. Chronic pain. Fatigue. Endless bouts of diarrhea. Days structured around restroom access. Relationships, careers, and joy are often sacrificed on the altar of a gut that has turned against its own body.
Despite decades of research, IBD—an umbrella term encompassing Crohn’s disease and ulcerative colitis—remains frustratingly hard to treat. Many therapies focus on taming inflammation, yet they offer only partial or temporary relief. The root causes of IBD’s persistent flare-ups remain stubbornly elusive.
But now, in a compelling new study published in Nature Metabolism, scientists may have uncovered a hidden cellular culprit—one that could change how we treat this debilitating disease. It’s not just inflammation that damages the gut lining. It might be a strange, fiery death of the cells themselves. A death called ferroptosis.
When Antioxidants Aren’t Enough
At first glance, the biology of IBD appears straightforward. Patients with IBD often show a surge in molecules known as reactive oxygen species (ROS) in their inflamed intestinal tissue. ROS are unstable, chemically reactive molecules—byproducts of normal cellular metabolism that, in excess, can ravage cells like molecular shrapnel.
This observation gave rise to a hopeful theory: if too much ROS is the problem, then antioxidants—molecules that neutralize ROS—could be the solution. In animal models of acute IBD, antioxidant treatments showed promise, calming inflammation and restoring function.
But then came the clinical trials in humans. Disappointing. Inconsistent. Sometimes even harmful.
The problem, it turns out, is that the story of ROS is not so black-and-white. “Newer research has revealed that too many antioxidants can cause what we call reductive stress,” explains Dr. Yatrik Shah, a physiologist at the University of Michigan Medical School and senior author of the new study. “That can cause dysfunction and kill cells, and also block signaling events that are important for normal cell function.”
In trying to put out one fire, researchers may have been unintentionally smothering essential biological signals. A different approach was needed—one that looked not just at the quantity of ROS, but at the type.
Searching for the Real Offender
In the Shah Lab, that question became personal. What if IBD isn’t caused by a general oxidative frenzy—but by a specific kind of ROS doing a very specific kind of damage?
Two graduate students in the lab, Wesley Huang and Yuezhong “Diana” Zhang, set out to find the answer. Working alongside colleagues like Dr. Peter Higgins in Gastroenterology at U-M, they analyzed intestinal tissue from both IBD patients and mice engineered to mimic chronic IBD. Their goal: detect fleeting ROS molecules in real-time, before they vanish.
What they found was startling.
In both mice and human samples, a specific type of ROS—lipid ROS—was unusually elevated in the intestinal epithelial cells lining the gut. These lipid-based oxidants aren’t just corrosive. They’re executioners.
Because when lipid ROS take over, they trigger a relatively newly discovered form of programmed cell death known as ferroptosis.
The Fire Within: Understanding Ferroptosis
Unlike other forms of cell death, such as apoptosis (which involves quiet self-destruction), ferroptosis is a fiery, iron-dependent meltdown. It floods cells with oxidative stress focused specifically on fat-containing membranes. Cells essentially rust from the inside out.
It’s a dramatic way for a cell to die—and it leaves behind a trail of inflammation and tissue damage.
What makes this relevant to IBD is not just that ferroptosis was happening. It was preferentially happening in IBD tissue—and not in healthy tissue.
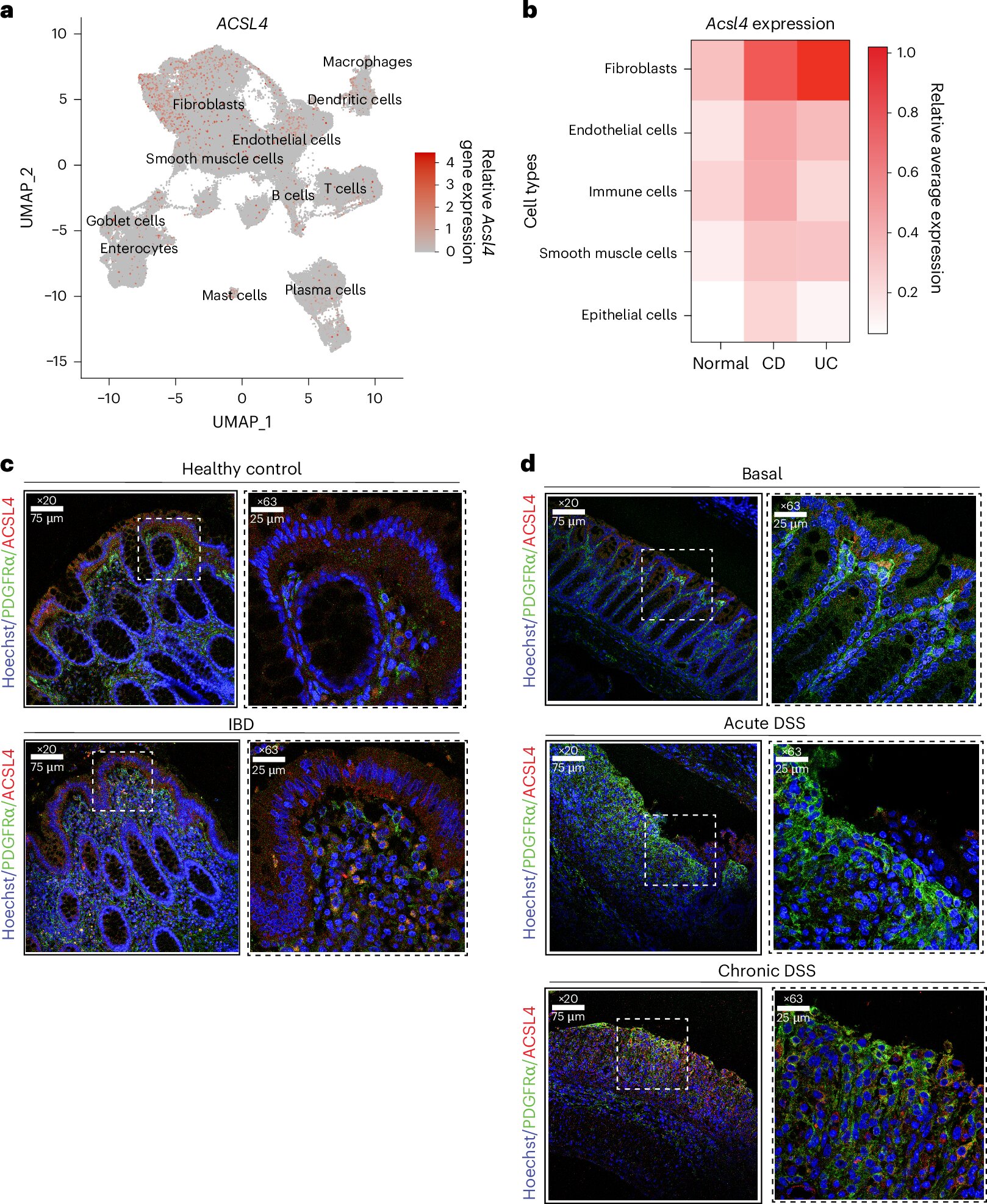
Digging deeper into gene expression data from patient biopsies, the team found increased activity in genes linked to ferroptosis. One gene in particular stood out: ACSL4, a molecule that plays a central role in creating the very lipids that make cells vulnerable to this unique death pathway.
When ACSL4 levels rise, so does the likelihood of ferroptosis.
The Smoking Gun in Fibroblasts
To confirm that ACSL4 was more than just a bystander, the researchers turned to their mouse models. There, they found something even more specific: the fibroblasts—cells that build connective tissue in the colon—were overexpressing ACSL4 in IBD models.
This overactivity, in turn, seemed to drive the generation of lipid ROS and trigger the deadly chain reaction of ferroptosis in nearby epithelial cells, which are critical for maintaining the gut barrier.
“Normal mice didn’t show the same effect,” Zhang noted, emphasizing that this destructive pattern only emerged in the presence of chronic inflammation like that seen in IBD. In other words, the cell death wasn’t just a passive side effect. It might be a driver of the disease.
Hope on the Horizon: A Target for New Therapies
If ferroptosis is fanning the flames of IBD, could turning it off ease the symptoms?
To find out, the researchers tested a drug that specifically inhibits ferroptosis. The results were promising. Treated mice showed relief from some of the worst symptoms of IBD. Their colons were less inflamed, the gut lining better preserved.
Even more tantalizing, targeting ACSL4 itself—a key player in the ferroptosis pathway—emerged as a potential therapeutic strategy. A drug that blocks ACSL4 activity might reduce the formation of lipid ROS without the drawbacks of broad-spectrum antioxidants.
As Shah put it, “If this is a major feature that drives the inflammatory nature of IBD and what makes the disease worse, there are ways to specifically target it. Instead of targeting all reactive oxidative species, we can just inhibit this one connected to ferroptosis.”
From Mystery to Medicine
This research does more than identify a new villain in the complex saga of IBD. It reframes how we think about inflammation, cell death, and oxidative stress. It reminds us that sometimes, the answer isn’t in stopping a process altogether, but in understanding its details—its chemistry, its triggers, its timing.
More importantly, it opens the door to precision medicine for diseases that desperately need it.
Because for someone living with Crohn’s or colitis, relief isn’t theoretical. It’s the ability to go through a day without pain. To work, travel, laugh—without fearing their own body.
By shining a spotlight on ferroptosis, the team at the University of Michigan may have just taken a major step toward making that future possible.
Reference: Wesley Huang et al, Fibroblast lipid metabolism through ACSL4 regulates epithelial sensitivity to ferroptosis in IBD, Nature Metabolism (2025). DOI: 10.1038/s42255-025-01313-x