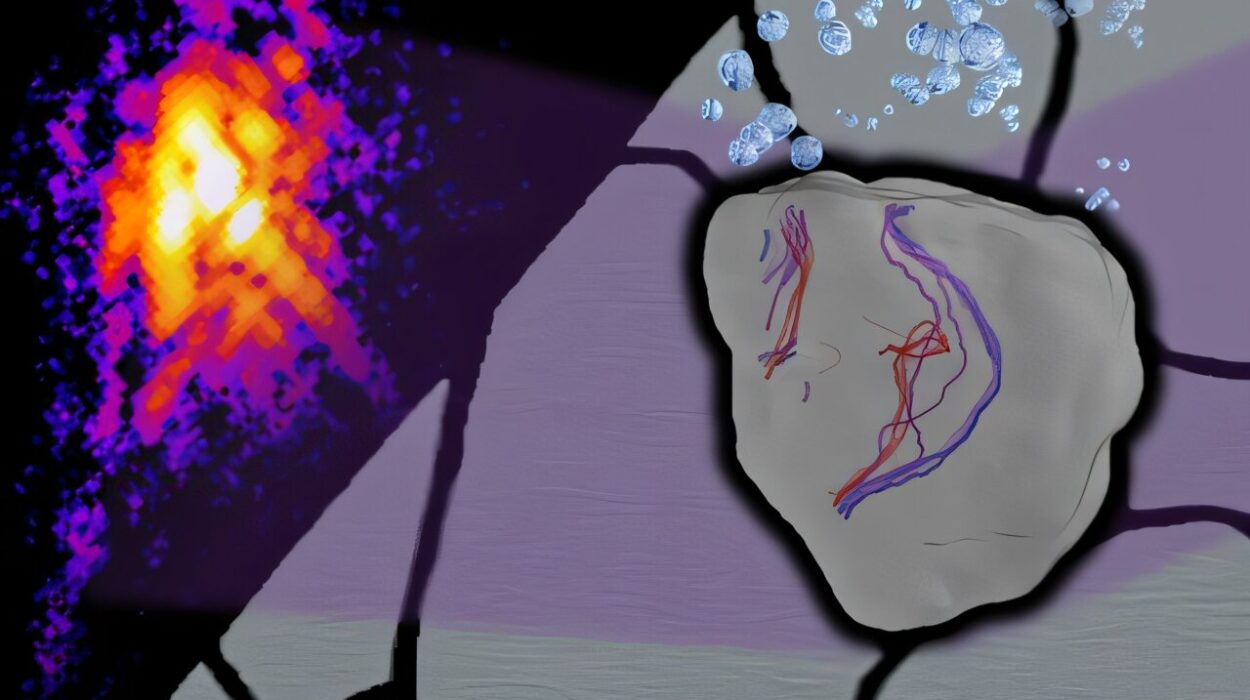
In a quiet corner of southern Spain, inside the laboratories of the University of Malaga, a team of chemists has illuminated a path toward the future of medicine—quite literally. Researchers from the Departments of Physical Chemistry and Organic Chemistry, working alongside scientists from the Biomimetic Dendrimers and Photonic Laboratory of IBIMA Plataforma BIONAND, have achieved something extraordinary: they have developed a new family of fluorescent molecules that shine brighter in the very environments where most others fade.
This remarkable finding, recently published in the prestigious journal Advanced Materials, marks a milestone at the intersection of materials science and biomedicine. It’s a story not only of chemistry but of creativity, curiosity, and the enduring human desire to see the unseen.
The Mystery of the Glowing Molecules
Fluorescent molecules—or dyes—are widely used in science to visualize the hidden worlds inside living cells. They act like lanterns, helping scientists trace molecular pathways, study cell structures, and even monitor disease progression. Yet, these luminous tools have long faced a stubborn problem: when placed in water or biological environments (such as the interior of a cell), they tend to dim.
This fading of brightness, known as “fluorescence quenching,” is a familiar frustration for researchers. But what the Malaga team discovered defies this expectation entirely. Their new molecules behave in what they call a “counterintuitive” way: instead of losing intensity when exposed to aqueous environments, they glow even brighter.
When dissolved in water or biological fluids, the molecules’ emission doesn’t weaken—it shifts toward the blue region of the light spectrum and intensifies. It’s as though the very conditions that normally silence a fluorescent signal instead amplify it, allowing these new compounds to shine with greater clarity and strength inside living systems.
The Science Behind the Glow
The beauty of this discovery lies in its paradox. Traditionally, fluorescent dyes are engineered to work best in dry or non-polar conditions, where their molecular structures remain stable. Water, with its polar nature, tends to disrupt these delicate interactions, scattering the light and reducing the intensity of fluorescence.
However, the molecules designed by the University of Malaga team seem to embrace water rather than fight it. Their structure encourages a phenomenon that enhances light emission under aqueous conditions. This means that, rather than flickering out when immersed in the cellular world, they reach their full radiance precisely when surrounded by the biological materials they are meant to study.
This surprising behavior not only challenges long-held assumptions in fluorescent chemistry but also expands the boundaries of how light-based imaging can be applied in medicine.
Lighting Up the Cell’s Powerhouse
One of the most exciting applications of these new fluorescent molecules lies in their affinity for mitochondria—the tiny, bean-shaped organelles often described as the “powerhouses” of the cell. Mitochondria are essential for producing the energy that keeps cells alive and functioning. They also play pivotal roles in aging, metabolism, cancer, and neurodegenerative diseases like Parkinson’s and Alzheimer’s.
The ability of these molecules to selectively target and illuminate mitochondria means that scientists can now capture detailed, high-contrast images of one of the most vital—and mysterious—structures in living cells. Using advanced imaging techniques such as multiphoton microscopy, researchers can explore these inner worlds with unprecedented precision and minimal harm to the cells themselves.
Multiphoton microscopy uses pulses of light to excite the fluorescent molecules deep within living tissues, generating sharp, three-dimensional images without causing the damage that traditional microscopy can inflict. This technique, combined with the new dyes, enables scientists to “photograph” life at the molecular level, revealing how mitochondria behave, interact, and change under different conditions.
Bridging Chemistry and Medicine
What makes this achievement particularly groundbreaking is its union of fundamental chemistry and applied biomedicine. These new fluorescent molecules are not only more effective but also simpler and more affordable to produce than many of their predecessors.
In practice, this could mean that laboratories around the world—especially those in developing regions—might soon gain access to high-quality imaging tools without the prohibitive costs associated with existing fluorescent dyes. The result is a democratization of cutting-edge biomedical imaging, empowering more scientists to investigate diseases and cellular processes in real time.
The researchers—led by Professors Ezequiel Pérez-Inestrosa, Carlos Benítez Martín, and Francisco Nájera Albendín, along with José Manuel Marín Beloqui, Juan T. López Navarrete, and Juan Casado Cordón—see this as more than just a technical success. It is a symbol of what can happen when curiosity-driven chemistry meets the real-world challenges of biology and medicine.
“These results are tremendously encouraging,” said Professors Pérez-Inestrosa and Casado. “Not only do these molecules challenge an established rule in fluorescent chemistry, but they also open the door to new tools for studying diseases where mitochondrial function is key. It is an example of what is achieved when fundamental chemistry meets research applied to biomedicine.”
Seeing the Future of Medicine
The implications of this discovery stretch far beyond the laboratory. In the near future, these molecules could revolutionize the way doctors diagnose and monitor diseases. Their ability to shine brightly in living tissues could enable earlier detection of disorders linked to mitochondrial dysfunction—such as cancer, metabolic syndromes, and neurodegeneration—before symptoms even appear.
Moreover, the affordability and efficiency of these dyes make them ideal candidates for large-scale biomedical applications. They could one day be used to guide precision treatments, visualize drug effects in real time, or even assist in minimally invasive surgeries where clear imaging is critical.
In a broader sense, the breakthrough represents the continuing evolution of biomedicine toward greater sensitivity, safety, and accessibility. As our understanding of the molecular world deepens, innovations like this remind us that the future of healthcare will not only rely on machines or algorithms but also on the brilliance of chemistry and the power of light.
The Beauty of the Unexpected
Science often advances when researchers dare to question what seems obvious. The Malaga team’s fluorescent molecules are a perfect example of that spirit. By embracing an unexpected result—molecules that glow brighter in water rather than dimming—they have redefined what is possible in a field that seemed already well understood.
It is a reminder that the natural world still holds countless surprises waiting to be uncovered, and that sometimes, progress begins not with certainty, but with curiosity and a willingness to follow where the light leads.
More information: Carlos Benitez‐Martin et al, Counterintuitive Fluorescence Blue Shift in Symmetry Breaking Dicationic Bis(indolium) with Two‐Photon Absorption Properties for NIR Living Cell Imaging, Advanced Materials (2025). DOI: 10.1002/adma.202510730